Microorganisms: Nature’s Tiny Chemists
Single-celled organisms, which include bacteria, archaea, some fungi, some algae, and most protists, are known as microorganisms, or microbes. Wherever there is water, there are microorganisms. Microorganisms, like other organisms, need water to live and reproduce. Microorganisms can be as small as a few microns in size (1 micron = 10−6 m) and can inhabit almost any nook or cranny you can think of, from at least 5 km beneath Earth’s surface to more than 10 km high in the atmosphere. They live in air, in soil, on and in rocks, inside roots, in piles of toxic waste, on frozen snowfields, and in water bodies of every type, including boiling hot springs. They live at temperatures that range from lower than −20°C to higher than the boiling point of water (100°C).
People have exploited the useful effects of microbial metabolism for thousands of years to produce bread, wine, and cheese. Today, people also use microorganisms to produce antibiotics and other valuable drugs. Geobiologists study microorganisms to understand their roles in biogeochemical cycles and to understand the early evolution of the biosphere before the advent of more complex organisms.
289
Abundance and Diversity of Microorganisms
Microorganisms dominate Earth in terms of numbers of individuals. Concentrations ranging from 103 to 109 microorganisms/cm3 have been reported from soils, sediments, and natural waters. Every time you walk on the ground, you step on billions of microorganisms! In some cases, surfaces become coated with dense concentrations of microorganisms called biofilms, which may contain as many as 108 individuals/cm2 of surface area.
More important, microorganisms are the most genetically diverse group of organisms on Earth. Genes are large molecules within the cells of every organism that encode all of the information that determines what that organism will look like, how it will live and reproduce, and how it differs from all other organisms. Genes are also the basic hereditary units passed on from generation to generation. The genetic diversity of microorganisms is important because it has allowed them to colonize, adapt to, and thrive in environments that would be lethal to most other organisms. These abilities, in turn, are important because they allow microorganisms to recycle important materials in a broad—even extreme—range of geologic environments.
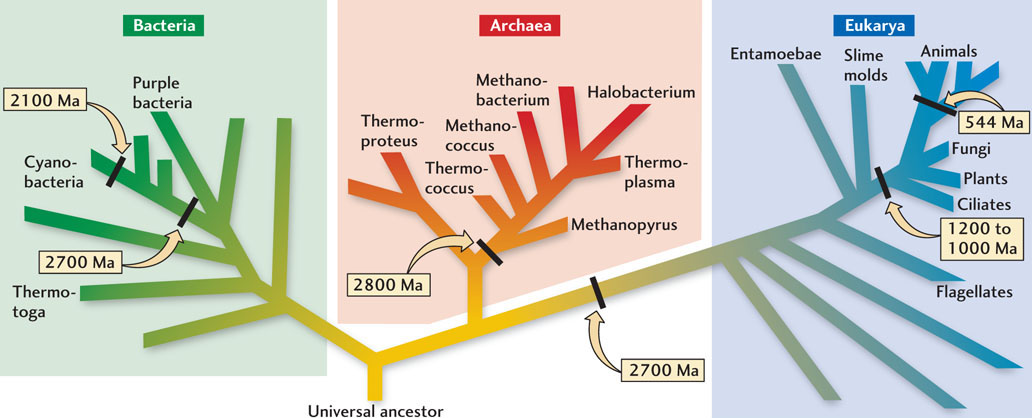
The Universal Tree of Life
Biologists have learned how to use the genetic information contained in living organisms to understand which forms of life are most closely related to one another. This knowledge has allowed them to organize the hierarchy of ancestors and descendants into a universal tree of life (Figure 11.5). About 30 years ago, a startling discovery was made when the first family trees for microorganisms were constructed. When the genes for all types of microorganisms were compared, it was shown that, despite their similar sizes (tiny) and shapes (simple rods and ellipses), there were enormous differences in their genetic content. Furthermore, when the genes of all types of organisms, including plants and animals, were compared, it was revealed that the differences among groups of microorganisms were much greater than the differences between plants and animals, including humans.
290
The Three Domains of Life
The single root of the universal tree of life shown in Figure 11.5 is called the universal ancestor. This universal ancestor gave rise to three major groups, or domains, of descendants: the Bacteria, the Archaea, and the Eukaryota. The Bacteria and Archaea appear to have evolved first; all of their descendants have remained single-celled microorganisms. The Eukaryota, thought to be the youngest branch of the tree, have a more complex cellular structure, which includes a cell nucleus that contains the genes. This structure made it possible for eukaryotes to evolve from small, single-celled organisms into larger, multicellular organisms—an essential step in the evolution of animals and plants.
Precambrian microorganisms, like those living today, were tiny. The traces of individual microorganisms preserved in rocks are therefore called microfossils. Needless to say, such features are much harder to find than the macroscopic fossils of shells, bones, and twigs used by geologists to study the evolution of animals and plants during the Phanerozoic eon (recall that Phanerozoic means “visible life”).
For geobiologists, the universal tree of life is a map that reveals how microorganisms relate to one another and interact with Earth. The names of microorganisms, such as Halobacterium, Thermococcus, and Methanopyrus, suggest that these organisms can live in extreme environments that are very salty (halo, “halite”), or hot (thermo), or high in methane (methano). Microorganisms that live in extreme environments are almost exclusively archaea and bacteria.
Extremophiles: Microorganisms That Live on The Edge
Extremophiles are microorganisms that live in environments that would kill other organisms (Table 11.3). The suffix phile is derived from the Latin word philus, which means “to have a strong affinity or preference for.” Extremophiles live on all kinds of foods, including oil and toxic wastes. Some use substances other than oxygen, such as nitric acid, sulfuric acid, iron, arsenic, or uranium, for respiration.
| Type | Tolerance | Environment | Example |
|---|---|---|---|
| Halophile | High salinity | Playa lakes Marine evaporites |
Great Salt Lake, Utah |
| Acidophile | High acidity | Mine drainage Water near volcanoes |
Rio Tinto, Spain |
| Thermophile | High temperature | Hot springs Mid-ocean ridge vents |
Yellowstone National Park |
| Anaerobe | No oxygen | Pores of wet sediments Groundwater Microbial mats Mid-ocean ridge vents |
Cape Cod Bay sediments |
Acidophiles are microorganisms that thrive in acidic environments. Acidophiles can tolerate pH levels low enough to kill other organisms. These microorganisms live by eating sulfide! They are able to survive in such acidic habitats because they have developed a way to pump out the acid that accumulates inside their cells. Such extremely acidic habitats occur naturally (see Earth Issues 11.1), but are more commonly associated with mining.
Thermophiles are microorganisms that live and grow in extremely hot environments. They grow best in temperatures that are between 50°C and 70°C and can tolerate temperatures up to 120°C. They will not grow if the temperature drops to 20°C. Thermophiles live in geothermal habitats, such as hot springs and hydrothermal vents at mid-ocean ridges, and in environments that create their own heat, such as compost piles and garbage landfills. The microorganisms that cover the bottom of Grand Prismatic Hot Spring (see the chapter opening photo) are dominated by thermophiles. Of the three domains of life, the Eukaryota (which include humans) are generally the least tolerant of high temperatures (60°C seems to be their upper limit). The Bacteria are more tolerant (with an upper limit close to 90°C), and the Archaea are the most tolerant, able to withstand temperatures of up to 120°C. Microorganisms that can stand temperatures above 80°C are called hyperthermophiles.
291
Halophiles are microorganisms that live and grow in highly saline environments. They can tolerate salt concentrations up to 10 times that of normal ocean water. Halophiles live in naturally hypersaline playa lakes such as the Great Salt Lake and the Dead Sea (see Chapter 19) and in some parts of the ocean, such as the southern end of San Francisco Bay, where seawater is commercially evaporated to extract salt (Figure 11.6). These microorganisms can control the salt concentration inside their cells by expelling extra salt from their cells into the environment.

Anaerobes are microorganisms that live in environments completely devoid of oxygen. At the bottoms of most lakes, streams, and oceans, the fluids in the pores of sediments just a few millimeters or centimeters below the sediment-water interface are starved of oxygen. Microorganisms that live at the sediment-water interface use up all the oxygen during respiration, creating an anaerobic (oxygen-free) zone beneath them in the sediment, where only anaerobes thrive. The oxygen-rich upper sediment layer is known as the aerobic zone. Many microorganisms that live in the aerobic zone could not survive in the anaerobic zone, and vice versa. The boundary between these zones is often very sharp, as shown in Figure 11.7.

292
Earth Issues: 11.1 Sulfide Minerals React to Form Acidic Waters on Earth and Mars
Many economically significant mineral deposits are associated with high concentrations of sulfide minerals. When water comes into contact with sulfide minerals, the sulfide they contain reacts with oxygen to form sulfuric acid. Thus, during the course of mining and afterward, rainwater and groundwater may interact with these minerals to produce highly acidic surface water and groundwater. Unfortunately, these acidic waters are lethal to most organisms. As they spread throughout the environment, extensive devastation may result. In some cases, the only organisms that survive are acidophilic extremophiles.
In a few places on Earth, where sulfide minerals occur in high enough concentrations, acidic waters are produced naturally. One of these places is the Rio Tinto in Spain. Here, geologists have been able to study a system in which a naturally occurring ore deposit, almost 400 million years old, interacts with groundwater that flows through it by hydrothermal circulation. With the help of mineral-dissolving acidophilic microorganisms, sulfide minerals such as pyrite (FeS2) in the ore deposit react with oxygen in the groundwater to produce sulfuric acid, sulfate ions (SO4−2), and iron ions (Fe3+). The warm spring water that flows out of the deposit as a river (rio in Spanish) is extremely acidic. Your skin would dissolve if you went swimming in that water.
The river is red (tinto in Spanish) because of the dissolved Fe3+ ions. The Fe3+ ions combine with oxygen to produce the iron oxide minerals goethite and hematite, which may be reddish or brownish in color. In addition, unusual iron sulfate minerals such as jarosite (yellow-brown in color) form abundantly in the Rio Tinto. When geologists encounter this mineral on Earth, they know that the water from which it precipitated must have been extremely acidic.
What is a rare—and environmentally damaging—geologic setting on Earth may once have been widespread on Mars. As we saw in Chapter 9, past exploration of Mars has revealed abundant sulfate minerals similar to those found in the Rio Tinto, including jarosite. Understanding how this unusual mineral forms on Earth allows geologists to make inferences about past environments on Mars. In this case, the presence of jarosite indicates that some ancient waters on Mars were very acidic, perhaps because of the interaction of groundwater with igneous rocks composed of basalt with trace amounts of sulfide.
This scenario has implications for how we think about the possibility of life—past or present—on other planets. Environments such as the Rio Tinto on Earth show that microorganisms have learned to adapt to highly acidic conditions, and they help motivate the search for ancient life on Mars. Some scientists, however, think that although life may have learned to adapt to such harsh conditions, it may not have been able to originate under those conditions. In any event, the search for life on other planets will be strongly guided by our understanding of rocks, minerals, and extreme environments on Earth.

Microorganism-Mineral Interactions
Microorganisms play a critical role in many geologic processes, including mineral precipitation, mineral dissolution, and the flux of elements through Earth’s crust in biogeochemical cycles. As we will learn later in this chapter, they have also been crucial factors in the evolutionary history of larger, more complex organisms.
Mineral Precipitation
Microorganisms precipitate minerals in two distinct ways: indirectly by influencing the composition of the water surrounding them and directly in their cells as a result of their metabolism. Indirect precipitation occurs when dissolved minerals in an oversaturated solution precipitate on the surfaces of individual microorganisms. This happens because the surface of a microorganism has sites that bind dissolved mineral-forming elements. Mineral precipitation often leads to the complete encrustation of the microorganisms, which are effectively buried alive. Microbial precipitation of carbonate minerals and silica in hot springs are good examples of this type of microbial biomineralization (Figure 11.8a). Thermophiles may become completely overgrown by the mineral deposits they help to precipitate.
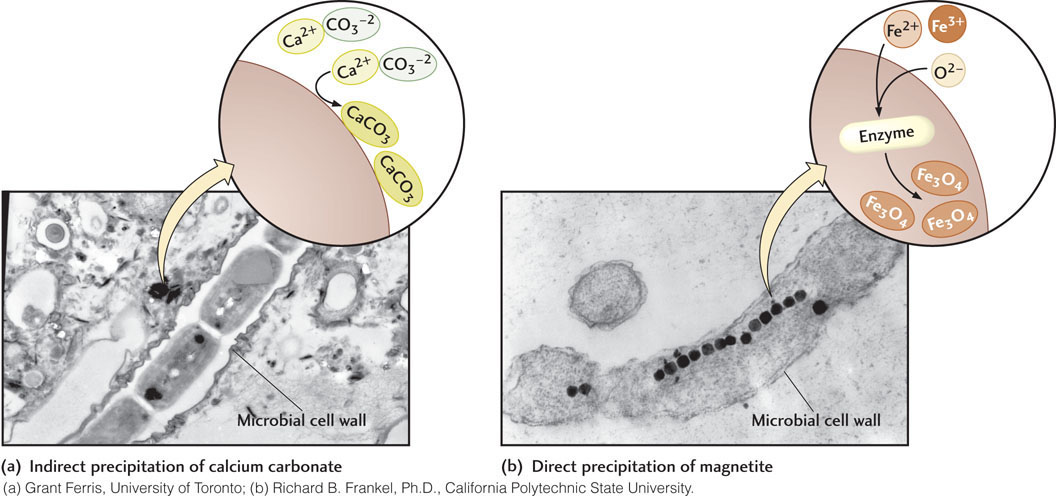
Minerals are directly precipitated by the metabolic activities of some microorganisms. For example, microbial respiration causes precipitation of pyrite (Figure 11.9) in the anaerobic zone of sediments that contain iron-bearing minerals and water in which sulfate is dissolved. As we have learned, all organisms—including microorganisms—need oxygen for respiration. In the anaerobic zone, however, O2 is not available. Some microbial decomposers have adapted to this harsh, but very common, environment by evolving ways to obtain oxygen from other sources. These microorganisms can remove the oxygen contained in sulfate (SO4), which is abundant in most sediment pore fluids. In the process, they make hydrogen sulfide gas (H2S), which produces the unpleasant odor of rotten eggs that is released when you dig into sandy or muddy sediments at low tide. In the final step of the process, hydrogen sulfide reacts with iron, which replaces the hydrogen to form pyrite (FeS2). Pyrite is remarkably abundant in sedimentary rocks that contain organic matter, such as shales. Another example of direct precipitation is the formation of tiny particles of magnetite inside some bacteria (Figure 11.8b), which use these crystals to navigate by sensing Earth’s magnetic field.
293
294
Mineral Dissolution
Some elements that are essential for microbial metabolism, such as sulfur and nitrogen, are readily available from natural waters in dissolved form, but others, such as iron and phosphorus, must be actively scavenged from minerals by microorganisms. All microorganisms need iron, but iron concentrations in near-surface waters are generally so low that the microorganisms must obtain it by dissolving nearby minerals. In a similar way, some microorganisms obtain phosphorus—required for construction of biologically important molecules—by dissolving minerals such as apatite (calcium phosphate). Some autotrophs derive their energy not from sunlight, but from the chemicals produced when minerals are dissolved. These organisms are known as chemoautotrophs (see Table 11.1). For example, manganese (Mn2+), iron (Fe2+), sulfur (S), ammonium (NH4+), and hydrogen (H2) supply microorganisms with energy when they are released from minerals.
Microorganisms dissolve minerals by producing organic molecules that react with those minerals to liberate ions from mineral surfaces. Rates of mineral dissolution are normally slow, but may be enhanced where minerals containing nutrient elements are coated by microbial biofilms. Mineral-dissolving acidophiles thrive in waters where mineral dissolution results in prolific acid formation.
Microorganisms and Biogeochemical Cycles
Pyrite precipitation by microorganisms plays an important role in the global biogeochemical cycling of sulfur (Figure 11.10). As we have seen, iron and sulfur are precipitated as pyrite, which accumulates abundantly within sediments. As layers of sediment are deposited, the pyrite becomes buried and encapsulated in sedimentary rocks. The pyrite remains buried until the rocks are returned to Earth’s surface by tectonic uplift. When the rocks are weathered, the iron and sulfur in the pyrite are dissolved as ions in water or become incorporated into new minerals that accumulate in sediments, starting the biogeochemical cycle over again.
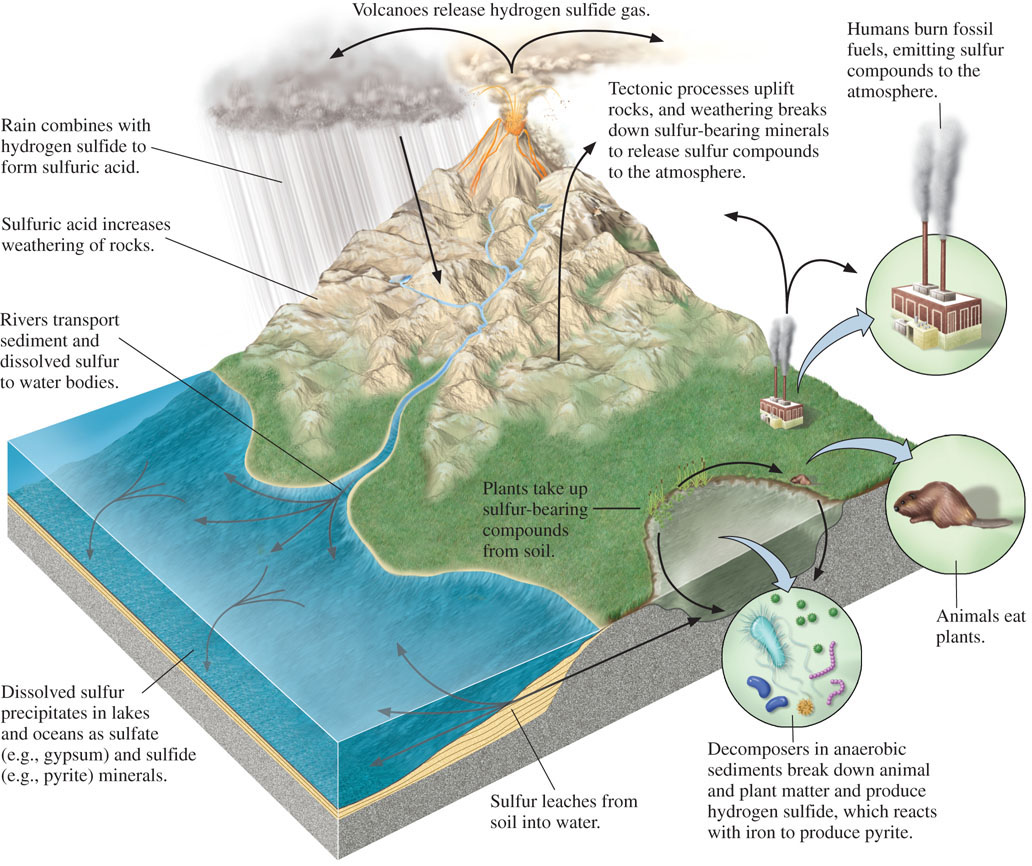
On a global scale, microorganisms play roles in several other biogeochemical cycles. Microbial precipitation of phosphate minerals contributes to the flow of phosphorus into sediments, particularly along the west coasts of South America and Africa, where phosphorus-rich deep ocean water that rises to the surface is available to microorganisms that live in shallower water, as we saw in Chapter 5. The chemical weathering of continental rocks is influenced by microorganisms that can increase the acidity of soils, leading to faster weathering rates. And finally, as we also saw in Chapter 5, the precipitation of carbonate minerals in marine environments is stimulated by microbial processes. This last example is especially important because carbonate minerals serve as a sink for atmospheric CO2 and for cations such as Ca2+ and Mg2+ released during weathering of silicate minerals.
Microbial Mats
Microbial mats are layered microbial communities. The microbial mats you are most likely to see are those that are exposed to the Sun (see Figure 11.7). They commonly occur in tidal flats, hypersaline lagoons, and hot springs. On the top, you will usually find a layer of oxygen-producing cyanobacteria that use energy from sunlight for photosynthesis. This uppermost layer is green because cyanobacteria contain the same light-absorbing pigment that plants and algae have. This layer may be as thin as 1 mm, yet it can be as effective in producing energy from the Sun as a hardwood forest or grassland. This uppermost green layer defines the aerobic zone of the mat. The anaerobic zone occurs below the cyanobacterial layer and is often a dark gray color. Although this anaerobic part of the mat contains no oxygen, it still can be very active. The anaerobic heterotrophs in this layer derive their food from the organic matter produced by the cyanobacteria. Their respiration often results in the precipitation of pyrite, as described earlier in this chapter.
Microbial mats are miniature models of the same biogeochemical cycles that occur at regional or even global scales. In a microbial mat, photosynthetic autotrophs use energy from sunlight to convert carbon in atmospheric CO2 into carbon in larger molecules such as carbohydrates. After the photoautotrophs die, the heterotrophs use the carbon in their bodies as an energy source. In the process, the heterotrophs convert some of this carbon into CO2, which is returned to the atmosphere, where it can be used by the next generation of photoautotrophs, and so on. In the case of microorganisms, this cycle is confined to the very small scale of a layer of sediment, but it is directly analogous to the process by which rain forests—at a global scale—extract CO2 from the atmosphere during photosynthesis. Although individual trees do the actual work, one can think of a rain forest as a giant photosynthesis machine that removes enormous quantities of CO2 from the atmosphere and produces enormous quantities of carbohydrates. When the trees die, their organic matter is used by heterotrophs on the forest floor to produce energy. This process returns enormous amounts of carbon—in the familiar form of CO2—to the atmosphere.
295
Stromatolites
Today, microbial mats are restricted to places on Earth where plants and animals cannot interfere with their growth. Before the evolution of plants and animals, however, microbial mats were widespread, and they are one of the most common features preserved in Precambrian sedimentary rocks formed in aquatic environments. Stromatolites—rocks with distinctive thin layers—are believed to have been formed from ancient microbial mats. Stromatolites range in shape from flat sheets to dome-shaped structures with complex branching patterns (Figure 11.11). They are one of the most ancient types of fossils on Earth and give us a glimpse of a world once ruled by microorganisms.
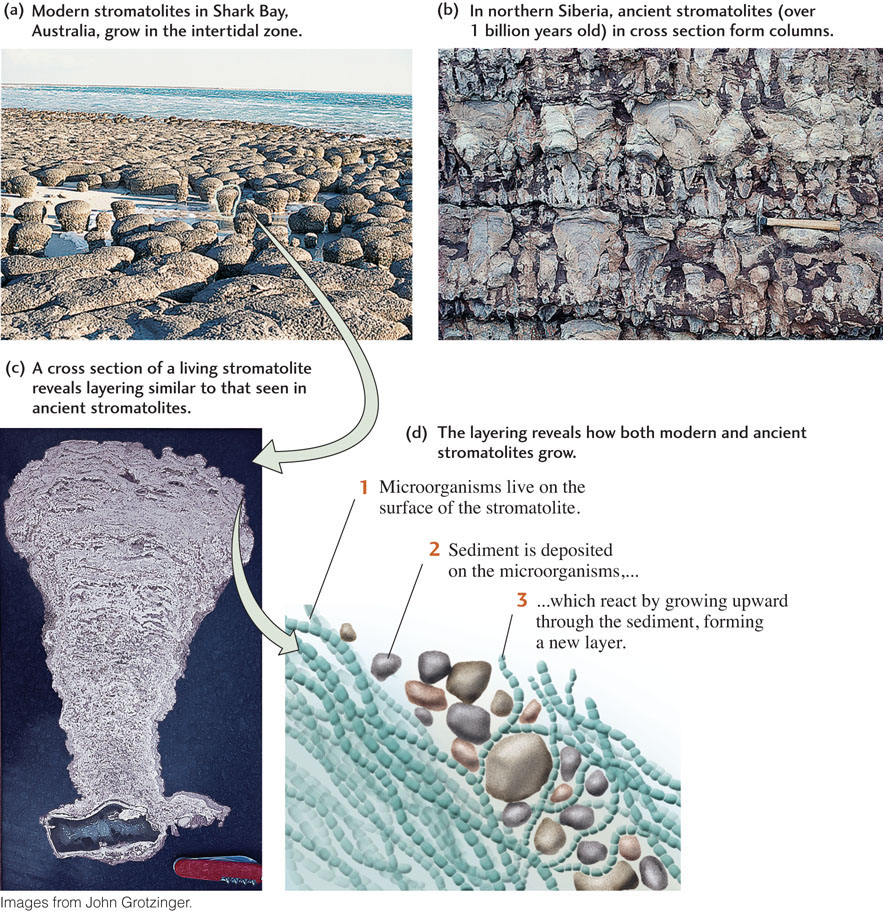
Most stromatolites probably formed when sediment raining down on microbial mats was trapped and bound by microorganisms living on the surfaces of the mats (Figure 11.11d). Once covered with sediment, the microorganisms grew upward between the sediment particles and spread laterally to bind the particles in place. Each stromatolite layer corresponds to the deposition of a sediment layer followed by the trapping and binding of that layer. Microbial communities can be observed building such structures today in intertidal environments such as Shark Bay, Western Australia (Figure 11.11a).
In other cases, however, stromatolites form by mineral precipitation, rather than by trapping and binding of sediment by microorganisms. That mineral precipitation may be indirectly controlled by microorganisms, or it may simply be the result of oversaturation of the surrounding water. As we saw in Chapter 5, the ocean contains abundant calcium and carbonate, which react to form the minerals calcite and aragonite. These minerals are important for the growth of stromatolites formed by mineral precipitation.
The potential role of microorganisms in stromatolite formation is important to understand because these layered, dome-shaped structures have been used as evidence for life on early Earth. But if stromatolites can be built by nonmicrobial mineral precipitation, their use as evidence for early life is uncertain. Only by carefully studying the processes by which microorganisms interact with minerals and sediments, and the chemical and textural fingerprints of these interactions, will we be able to determine whether the formation of stromatolites on early Earth required the presence of microorganisms.
296