Practicing Geology Exercise
How Do Geobiologists Find Evidence of Early Life in Rocks?
Perhaps the most important question a geobiologist can ask is, “What evidence of life is preserved in rocks?” If fossils of animal shells and skeletons are present in rocks, then this question is easy to answer. In many cases, however, the geologic processes that turn sediments into sedimentary rocks destroy the materials that could have become fossils. Furthermore, most organisms do not have hard shells or skeletons that are easily preserved, so we would not expect them to become fossils. And on early Earth, before the advent of animals with hard shells and skeletons, most organisms were microscopic in size. Simply put, how can the former presence of life on Earth be detected in situations in which fossils are not preserved?
One approach that geobiologists often depend on is the use of chemical signatures of former life. Carbon provides the most obvious example of an element that might have been concentrated by biological processes. Not all concentrations of carbon are of biological origin, however, so additional tests must be applied.
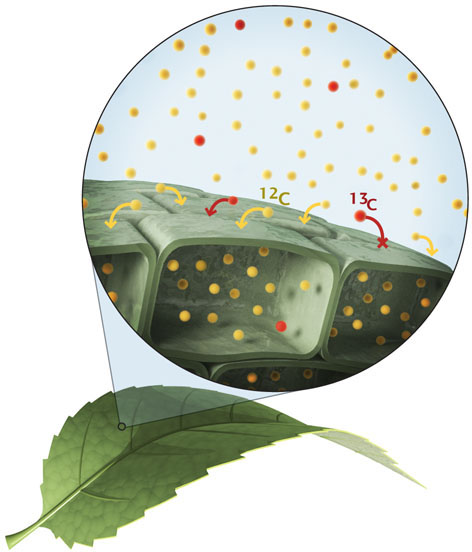
One of these tests asks whether the carbon present has a distinctive isotopic composition. Recall from Chapter 3 that isotopes are atoms of the same element with different numbers of neutrons. Many elements of low atomic weight have two or more stable (nonradioactive) isotopes. A carbon atom has six protons, but may have six, seven, or eight neutrons, which give it atomic masses of 12, 13, or 14, respectively. Carbon-12 is by far the most common isotope, so samples of carbon from ancient rocks or modern sediments will yield mostly carbon-12.
Fortunately, it turns out that metabolic processes such as photosynthesis use carbon-12 and carbon-13 differently. The difference in atomic mass between carbon-12 (often denoted 12C) and carbon-13 (13C) results in differences in their uptake by organisms. Photosynthetic organisms, for example, use carbon dioxide and water to form carbohydrates. They use carbon dioxide molecules that contain 12C preferentially over those that contain 13C. As a result, photosynthetic organisms become enriched in 12C relative to the environment from which they draw carbon dioxide.
We can therefore use carbon isotopes as a tool to detect ancient life by measuring the amounts of 12C and 13C present in sedimentary rocks. If sediments are formed in the presence of organic matter that is enriched in 12C (or in any particular isotope), this enrichment may be passed along to the sediments, and then on to the resulting rock. Thus, a shale that might be billions of years old can preserve a “signature” of life recorded by its carbon isotope composition.
We begin by measuring the amounts of 12C and 13C in a rock sample and calculating the ratio between them (12C/13C). We then compare that ratio with the 12C/13C ratio in a standard. The standard is a material (often a pure mineral, such as calcite) whose 12C/13C ratio is precisely known and varies very little. The standard can be compared over and over again with samples of other carbon-bearing rocks and sediments, as well as with living organisms and other natural substances. By comparing both rock samples and organisms with the standard, we can search for similarities that link rock samples to particular biological processes.
The following table gives the 12C/13C ratios for a standard, three rock samples, and two natural substances—plant material and methane gas:

The following equation* allows us to compare these data:
R(sample) = [12C/13C of the standard] − [12C/13C of the sample]
where R stands for the value of the difference between the two ratios.
For most rocks, the value of R will be close to zero, but could be slightly positive or negative. In contrast, if photosynthesis was involved in the formation of organic matter incorporated into a sedimentary rock—for example, a shale—the value of R for a sample of that shale could be very negative—close to −20. Some chemoautotrophic microorganisms that consume methane gas produce carbonate rocks with an extremely negative R value, on the order of −50.
311
Using the data and equation above, let’s try to identify which of our rock samples formed in the presence of biological processes. Let’s begin with rock B:

This result shows that rock B has an R value that is substantially different from zero, suggesting that ancient biological processes may have been at work when the rock formed. We can confirm that its value of −20 is a close match for the R value predicted for photosynthesis by calculating the R value for plant material:
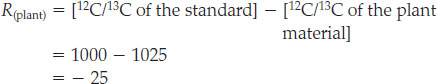
The R value for the plant material, at −25, is close enough to the R value for rock B, at −20, that our hypothesis of ancient photosynthesis is strengthened.
BONUS PROBLEM: Try calculating R values for rocks A and C. Which rock does not record a distinctive signature of biological processes? Is there a rock among our samples that might have formed in the presence of methane-consuming microorganisms? If so, how can you check this result?