Water Quality
Unlike people in many other parts of the world, North Americans are fortunate in that almost all of their public water supplies are free of bacterial contamination, and the vast majority are free enough of chemical contaminants to drink safely. Yet as more rivers become polluted and more aquifers are contaminated by toxic wastes, North Americans are likely to see changes in water quality. Most residents of the United States are beginning to see their supply of fresh, pure water as a limited resource. Many people now travel with their own supply of bottled water, supplied by either home-installed purification systems or commercially available spring water.
Contamination of the Water Supply
The quality of groundwater is often threatened by a variety of contaminants. Most of these contaminants are chemicals, though microorganisms in water can also have negative effects on human health under certain conditions.
Lead Pollution
Lead is a well-known pollutant produced by industrial processes that inject contaminants into the atmosphere. When water vapor condenses in the atmosphere, lead is incorporated into precipitation, which then transports it to Earth’s surface. Lead is routinely eliminated from public water supplies by chemical treatment before the water is distributed through the water mains. In older homes with lead pipes, however, lead can leach into the water. Even in newer construction, lead solder used to connect copper pipes and metals used in faucets may be sources of contamination. Replacing old lead pipes with durable plastic pipes can reduce lead contamination. Even letting the water run for a few minutes to clear the pipes can help.
Other Chemical Contaminants
A number of human activities produce chemicals that can contaminate groundwater (Figure 17.23). Some decades ago, when we knew much less about the health and environmental effects of toxic wastes, industrial, mining, and military wastes now known to be hazardous were dumped on the ground, disposed of in lakes and streams, or discharged underground. Even though many of these sources of pollution are being addressed, the contaminants are still making their way through aquifers by the slow flow of groundwater, and toxic chemicals are still entering groundwater from a number of other sources.
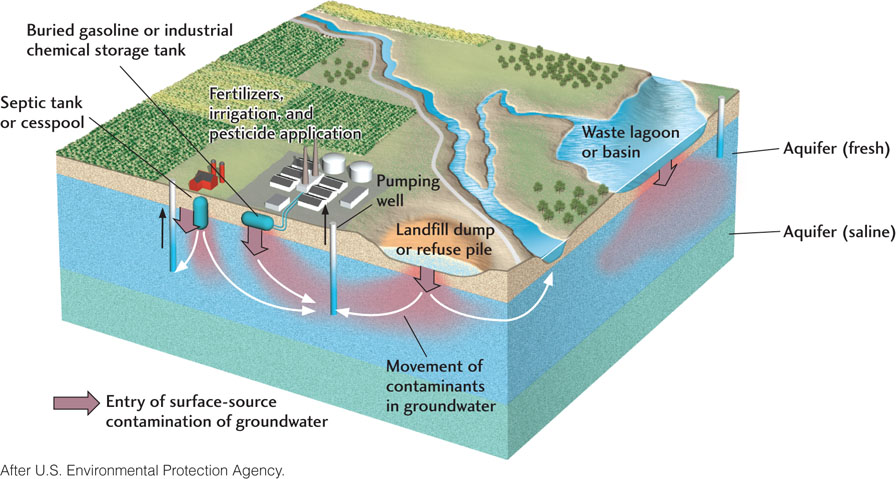
The disposal of chlorinated solvents—such as trichloroethylene (TCE), widely used as a cleaner in industrial processes—poses a formidable problem. These solvents persist in the environment because they are difficult to remove from contaminated waters. The burning of coal and the incineration of municipal and medical waste emit mercury into the atmosphere, which then contaminates water supplies. Buried gasoline storage tanks can leak, and road salt inevitably drains into the soil and ultimately into aquifers. Rain can wash agricultural pesticides, herbicides, and fertilizers into the soil, from which they percolate downward into aquifers. In some agricultural areas where nitrate fertilizers are heavily used, groundwaters contain high concentrations of nitrate. In one recent study, 21 percent of the shallow wells sampled exceeded the maximum amounts of nitrate (10 ppm) allowed in drinking water in the United States. Such high nitrate levels pose a danger of “blue baby” syndrome (an inability to maintain healthy oxygen levels) to infants 6 months old and younger.
Radioactive Wastes
There is no easy solution to the problem of groundwater contamination by radioactive wastes. When radioactive wastes are buried underground, they may be leached by groundwater and find their way into aquifers. Storage tanks and burial sites at the atomic weaponry plants in Oak Ridge, Tennessee, and Hanford, Washington, have already leaked radioactive wastes into shallow groundwaters.
Microorganisms
The most widespread causes of groundwater contamination by microorganisms are leaky residential septic tanks and cesspools. These containers, widely used in neighborhoods that lack full sewer networks, are buried settling tanks in which bacteria decompose the solid wastes from household sewage. To prevent contamination of drinking water, cesspools should be replaced by septic tanks, which must be installed at sufficient distance from water wells in shallow aquifers.
491
Reversing Contamination
Can we reverse the contamination of groundwater supplies? Yes, but the process is costly and very slow. The faster an aquifer recharges, the easier it is to decontaminate. If the recharge rate is rapid, fresh water moves into the aquifer as soon as we close off the sources of contamination, and in a relatively short time, the water quality is restored. Even a fast recovery, however, can take a few years.
The contamination of slowly recharging aquifers is more difficult to reverse. The rate of groundwater movement may be so slow that contamination from a distant source takes a long time to appear. By the time it does, it is too late for rapid remediation. Even after the recharge area has been cleaned up, some contaminated deep aquifers extending hundreds of kilometers from the recharge area may not be free of contaminants for many decades.
When public water supplies are polluted, we can pump the water and then treat it chemically to make it safe, but that is an expensive procedure. Alternatively, we can try to treat the water while it remains underground. In one moderately successful experimental procedure, contaminated water was funneled into a buried bunker full of iron filings, which detoxified the water by reacting with the contaminants. The reactions produced new, nontoxic compounds that attached themselves to the iron filings.
Is the Water Drinkable?
Much of the water in groundwater reserves is unusable not because it has been contaminated by human activities, but because it naturally contains large quantities of dissolved materials. Water that tastes agreeable and is not dangerous to human health is called potable water. The amounts of dissolved materials in potable waters are very small, usually measured by weight in parts per million (ppm). Potable groundwaters of good quality typically contain about 150 ppm total dissolved materials because even the purest natural waters contain some dissolved substances derived from weathering. Only distilled water contains less than 1 ppm dissolved materials.
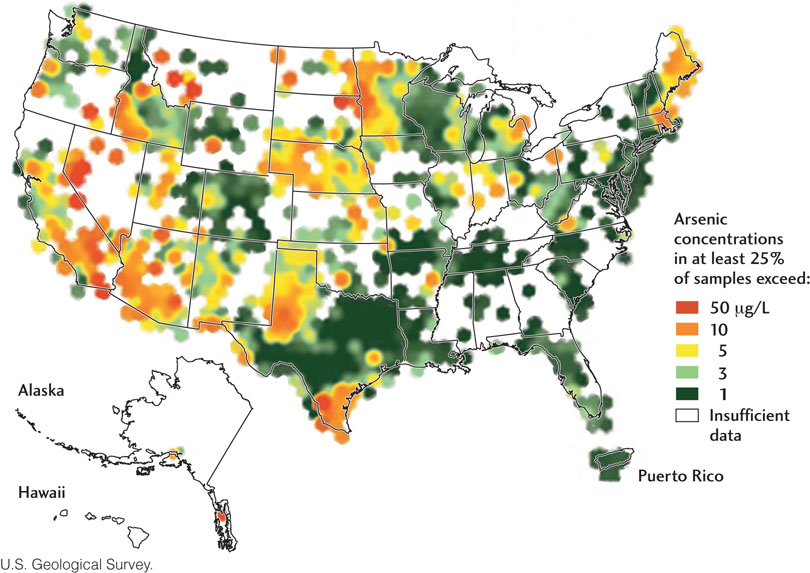
Geologic studies of streams and aquifers allow us to improve the quality of our water resources as well as their quantity. The many cases of groundwater contamination caused by human activity have led to the establishment of water quality standards based on medical studies. These studies have concentrated on the effects of ingesting average amounts of water containing various quantities of contaminants, both natural and anthropogenic. For example, the U.S. Environmental Protection Agency has set the maximum allowable concentration of arsenic, a well-known poison, at 0.05 ppm (Figure 17.24). Natural contamination of groundwater by arsenic is particularly acute in Bangladesh, where groundwater provides 97 percent of the drinking water supply. Geologists are helping to guide the placement of new wells that draw water with acceptable concentrations of arsenic.
Groundwater is almost always free of solid particles when it seeps into a well from a sand or sandstone aquifer. The complex passageways of the pore networks in the rock or sand act as a fine filter, removing small particles of clay and other solids and even straining out microorganisms and some large viruses. Limestone aquifers may have larger pores and so may filter water less efficiently. Any microbial contamination found at the bottom of a well is usually introduced from nearby underground sewage disposal systems, often when septic tanks leak or are located too close to the well.
492
Some groundwaters, although perfectly safe to drink, simply taste bad. Some have a disagreeable taste of “iron” or are slightly sour. Groundwaters passing through limestone dissolve carbonate minerals and carry away calcium, magnesium, and bicarbonate ions, making the water “hard.” Hard water may taste fine, but it does not lather readily when used with soap. Water passing through waterlogged forests or swampy soils may contain dissolved organic compounds and hydrogen sulfide, which give the water a disagreeable smell similar to rotten eggs.
How do these differences in taste and quality arise in safe drinking waters? Some of the highest-quality, best-tasting public water supplies come from lakes and artificial surface reservoirs, many of which are simply collecting places for rainwater. Some groundwaters taste just as good; these tend to be waters that pass through rocks that weather only slightly. Sandstones made up largely of quartz, for example, contribute little in dissolved materials, and thus waters passing through them have a pleasant taste.
As we have seen, the contamination of groundwater in relatively shallow aquifers is a serious problem, and remediation is difficult. But are there deeper groundwaters that we can use?