Water Deep in the Crust
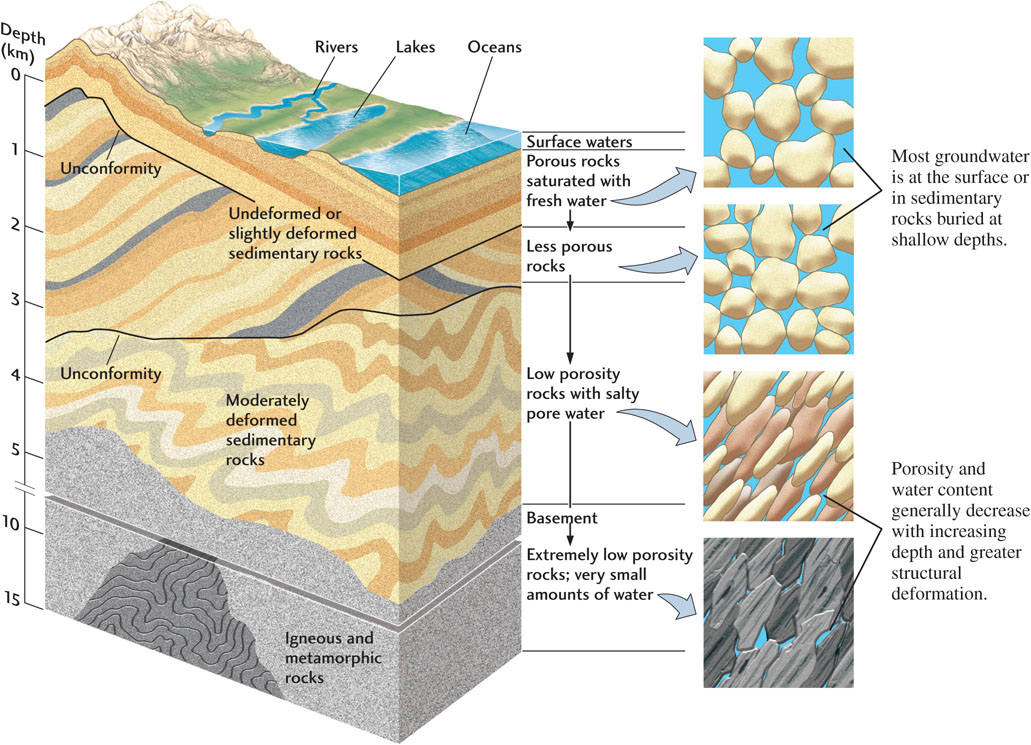
Most crustal rocks below the groundwater table are saturated with water. Even in the deepest wells drilled for oil, some 8 or 9 km deep, geologists find water in permeable formations. At these depths, groundwaters move so slowly—probably less than a centimeter per year—that they have plenty of time to dissolve minerals from the rocks through which they pass. Thus, dissolved materials become more concentrated in these waters than in near-surface waters, making them unpotable. For example, deep groundwaters that pass through salt beds, which dissolve quickly, tend to contain large concentrations of sodium chloride.
At depths greater than 12 to 15 km, deep in the basement igneous and metamorphic rocks that underlie the sedimentary formations of the upper crust, porosities and permeabilities are very low due to the tremendous weight of the overlying rocks. Although these rocks contain very little water, they are saturated (Figure 17.25). Even some mantle rocks are presumed to contain water, although in minute quantities.
Hydrothermal Waters
In some regions of the crust, such as along subduction zones, hot waters containing dissolved carbon dioxide play an important role in the chemical reactions of metamorphism, as we saw in Chapter 6. These hydrothermal waters dissolve some minerals and precipitate others.
Most hydrothermal waters of the continents come from meteoric waters that percolate downward to deeper regions of the crust. The percolation rates for meteoric waters deep in the crust are very low, and thus the water may be very old. It has been determined that the water at Hot Springs, Arkansas, derives from rain and snow that fell more than 4000 years ago and slowly infiltrated the ground. Water that escapes from magma can also contribute to hydrothermal waters. In areas of igneous activity, sinking meteoric waters are heated as they encounter hot masses of rock. The hot meteoric waters then mix with water released from the nearby magma.
493
Hydrothermal waters are loaded with chemical substances dissolved from rocks at high temperatures. As long as the water remains hot, the dissolved material stays in solution. However, as hydrothermal waters reach the surface, where they cool quickly, they may precipitate various minerals, such as opal (a form of silica) and calcite or aragonite (forms of calcium carbonate). Crusts of calcium carbonate produced at some hot springs build up to form the rock travertine, which can form impressive deposits such as those seen at Mammoth Hot Spring in Yellowstone National Park (Figure 17.26). Amazingly, microbial extremophiles that can withstand temperatures above the boiling point of water have been discovered in these environments, where they may contribute to the formation of calcium carbonate crusts. Hydrothermal waters that cool slowly below the surface deposit some of the world’s richest metallic ores, as we learned in Chapter 3.

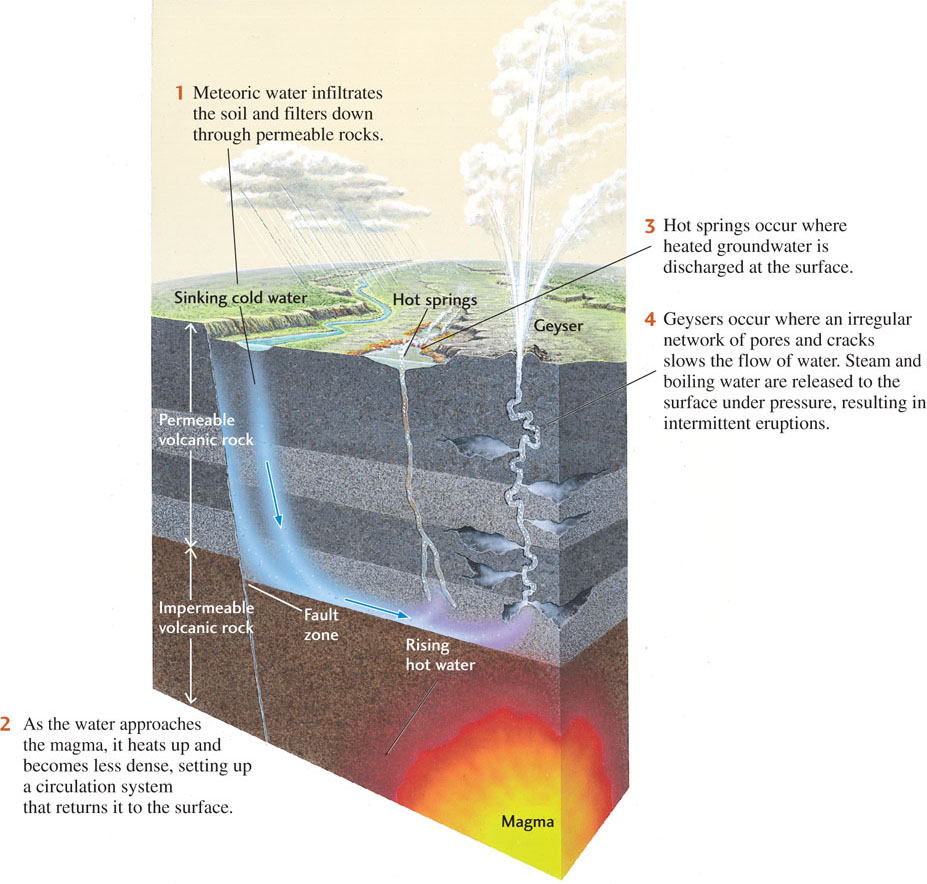
Hot springs and geysers exist where hydrothermal waters migrate rapidly upward without losing much heat and emerge at the surface, sometimes at boiling temperatures. Hot springs flow steadily; geysers erupt hot water and steam intermittently (see Figure 12.21).
The theory explaining the intermittent eruption of geysers is an example of geologic deduction. We cannot observe the process directly because the dynamics of underground hydrothermal systems are hidden from sight hundreds of meters below the surface. Geologists hypothesized that geysers are connected to the surface by a system of very irregular and crooked fractures, recesses, and openings, in contrast to the more regular and direct plumbing of hot springs (Figure 17.27). The irregular fractures sequester some water in recesses, thus helping to prevent the deepest waters from mixing with shallower waters and cooling. The deepest waters are heated by contact with hot rock. When they reach the boiling point, steam starts to ascend and heats the shallower waters, increasing the pressure and triggering an eruption. After the pressure is released, the geyser becomes quiet as the fractures slowly refill with water.
494
In 1997, geologists reported the results of a novel technique used to study geysers. They lowered a miniature video camera to about 7 m below the surface of a geyser. They found that the geyser shaft was constricted at that point. Farther down, the shaft widened to a large chamber containing a wildly boiling mixture of steam, water, and what appeared to be carbon dioxide bubbles. These direct observations dramatically confirmed the previous theory of how geysers work.
Although hydrothermal waters are useful to human society as sources of geothermal energy and metallic ores, these waters do not contribute to surface water supplies, primarily because they contain so much dissolved material.
Ancient Microorganisms in Deep Aquifers
In recent years, geologists have explored aquifers deep underground (as much as several thousand meters) in search of potable groundwater. They failed to find it, but they did unveil a remarkable interaction between the biosphere and the lithosphere. They found microorganisms living in the groundwater in huge numbers. These chemoautotrophic microorganisms, well out of the reach of sunlight, derive their energy by dissolving and metabolizing minerals in rocks. These metabolic reactions, aside from serving as a source of energy for the microorganisms, continue the weathering process underground. The chemicals released by these reactions make the water unpotable.
Geobiologists think that the ancestors of these microorganisms were enclosed within the pores of sediments, which were then buried at great depths, where they became sealed off from the surface. In some cases, these deep aquifers may not have been in contact with Earth’s surface for hundreds of millions of years. Yet the microorganisms persisted, living solely on chemicals provided by the dissolution of minerals and evolving new generations of descendants without interference from any other organisms. These ecosystems, involving only microorganisms, are probably the most ancient on Earth and testify to the remarkable balance that can be achieved between life and environment.