Peeling the Onion: Discovery of a Layered Earth
Ancient thinkers divided the universe into two parts, the heavens above and Hades below. The sky was transparent and full of light, and they could directly observe its stars and track its wandering planets. But Earth’s interior was dark and closed to human view. In some places, the ground quaked and erupted hot lava. Surely something terrible was going on down there!
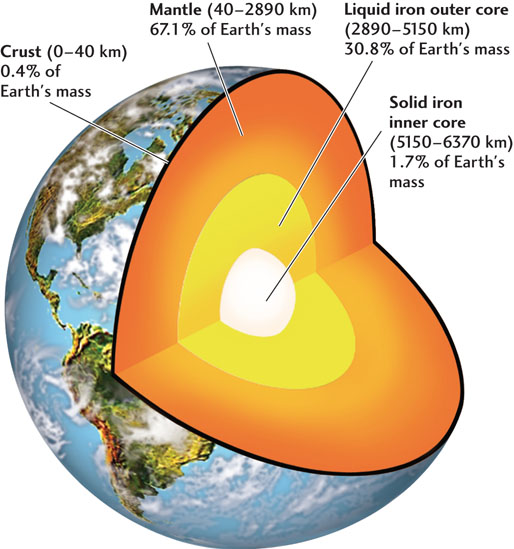
So it remained until about a century ago, when geologists began to peer downward into Earth’s interior, not with waves of light (which cannot penetrate rock), but with waves produced by earthquakes. An earthquake occurs when geologic forces cause brittle rocks to fracture, sending out vibrations like the cracking of ice on a river. These seismic waves (from the Greek word for earthquake, seismos), when recorded on sensitive instruments called seismometers, allow geologists to locate earthquakes and also to make pictures of Earth’s inner workings, much as doctors use ultrasound and CAT scans to image the inside of your body. When the first networks of seismographs were installed around the world at the end of the nineteenth century, geologists began to discover that Earth’s interior was divided into concentric layers of different compositions, separated by sharp, nearly spherical boundaries (Figure 1.9).
10
Earth’s Density
Layering of Earth’s deep interior was first proposed by the German physicist Emil Wiechert at the end of the nineteenth century, before much seismic data had become available. He wanted to understand why our planet is so heavy, or more precisely, so dense. The density of a substance is easy to calculate: just measure its mass on a scale and divide by its volume. A typical rock, such as the granite used for tombstones, has a density of about 2.7 grams per cubic centimeter (g/cm3). Estimating the density of the entire planet is a little harder, but not much. Eratosthenes had shown how to measure Earth’s volume in 250 b.c., and sometime around 1680, the great English scientist Isaac Newton figured out how to calculate its mass from the gravitational force that pulls objects to its surface. The details, which involved careful laboratory experiments to calibrate Newton’s law of gravity, were worked out by another Englishman, Henry Cavendish. In 1798, he calculated Earth’s average density to be about 5.5 g/cm3, twice that of tombstone granite.
Wiechert was puzzled. He knew that a planet made entirely of common rocks could not have such a high density. Most common rocks, such as granite, contain a high proportion of silica (silicon plus oxygen; SiO2) and have relatively low densities, below 3 g/cm3. Some iron-rich rocks brought to Earth’s surface by volcanoes have densities as high as 3.5 g/cm3, but no ordinary rock approached Cavendish’s value. He also knew that, going downward into Earth’s interior, the pressure on rock increases with the weight of the overlying mass. The pressure squeezes the rock into a smaller volume, making its density higher. But Wiechert found that even the effect of pressure was too small to account for the density Cavendish had calculated.
The Mantle and Core
In thinking about what lay beneath his feet, Wiechert turned outward to the solar system and, in particular, to meteorites, which are pieces of the solar system that have fallen to Earth. He knew that some meteorites are made of an alloy (a mixture) of two heavy metals, iron and nickel, and thus have densities as high as 8 g/cm3 (Figure 1.10). He also knew that these two elements are relatively abundant throughout our solar system. So, in 1896, he proposed a grand hypothesis: sometime in Earth’s past, most of the iron and nickel in its interior had dropped inward to its center under the force of gravity. This movement created a dense core, which was surrounded by a shell of silicate-rich rock that he called the mantle (using the German word for “coat”). With this hypothesis, he could come up with a two-layered Earth model that agreed with Cavendish’s value for Earth’s average density. He could also explain the existence of iron-nickel meteorites: they were chunks from the core of an Earthlike planet (or planets) that had broken apart, most likely by collision with other planets.

Wiechert got busy testing his hypothesis using seismic waves recorded by seismographs located around the globe (he designed one himself). The first results showed a shadowy inner mass that he took to be the core, but he had problems identifying some of the seismic waves. These waves come in two basic types: compressional waves, which expand and compress the material they move through as they travel through a solid, liquid, or gas; and shear waves, which move the material from side to side. Shear waves can propagate only through solids, which resist shearing, and not through fluids (liquids or gases) such as air and water, which have no resistance to this type of motion.
In 1906, a British seismologist, Robert Oldham, was able to sort out the paths traveled by these two types of seismic waves and show that shear waves did not propagate through the core. The core, at least in its outer part, was liquid! This finding turns out to be not too surprising. Iron melts at a lower temperature than silicates, which is why metallurgists can use containers made of ceramics (which are silicate materials) to hold molten iron. Earth’s deep interior is hot enough to melt an iron-nickel alloy, but not silicate rock. Beno Gutenberg, one of Wiechert’s students, confirmed Oldham’s observations and, in 1914, determined that the depth of the core-mantle boundary was about 2890 km (see Figure 1.9).
11
The Crust
Five years earlier, a Croatian scientist had detected another boundary at the relatively shallow depth of 40 km beneath the European continent. This boundary, named the Mohorovičić discontinuity (Moho for short) after its discoverer, separates a crust composed of low-density silicates, which are rich in aluminum and potassium, from the higher-density silicates of the mantle, which contain more magnesium and iron.
Like the core-mantle boundary, the Moho is a global feature. However, it was found to be substantially shallower beneath the oceans than beneath the continents. On average, the thickness of oceanic crust is only about 7 km, compared with almost 40 km for continental crust. Moreover, rocks in the oceanic crust contain more iron, and are therefore denser, than continental rocks. Because continental crust is thicker but less dense than oceanic crust, the continents ride higher by floating like buoyant rafts on the denser mantle (Figure 1.11), much as icebergs float on the ocean. Continental buoyancy explains the most striking feature of Earth’s surface topography: why the elevations shown in Figure 1.8 fall into two main groups, 0 to 1 km above sea level for much of the land surface and 4 to 5 km below sea level for much of the deep sea.
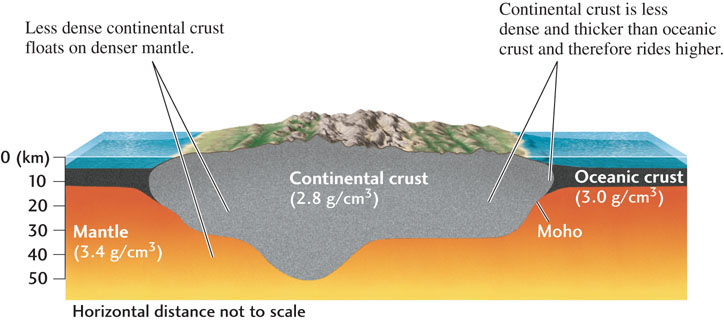
Shear waves travel well through the mantle and crust, so we know that both are solid rock. How can continents float on solid rock? Rock can be solid and strong over the short term (seconds to years), but weak over the long term (thousands to millions of years). The mantle below a depth of about 100 km has little strength, and over very long periods, it flows as it adjusts to support the weight of continents and mountains.
The Inner Core
Because the mantle is solid and the outer part of the core is liquid, the core-mantle boundary reflects seismic waves, just as a mirror reflects light waves. In 1936, Danish seismologist Inge Lehmann discovered another sharp spherical boundary at the much greater depth of 5150 km, indicating a central mass with a higher density than the liquid core. Studies following her pioneering research showed that the inner core can transmit both shear waves and compressional waves. The inner core is therefore a solid metallic sphere suspended within the liquid outer core—a “planet within a planet.” The radius of the inner core is 1220 km, about two-thirds the size of the Moon.
12
Geologists were puzzled by the existence of this “frozen” inner core. They knew that temperatures inside Earth should increase with depth. According to the best current estimates, Earth’s temperature rises from about 3500°C at the core-mantle boundary to almost 5000°C at its center. If the inner core is hotter, how could it be solid while the outer core is molten? The mystery was eventually solved by laboratory experiments on iron-nickel alloys, which showed that the “freezing” was due to higher pressures, rather than lower temperatures, at Earth’s center.
Chemical Composition of Earth’s Major Layers
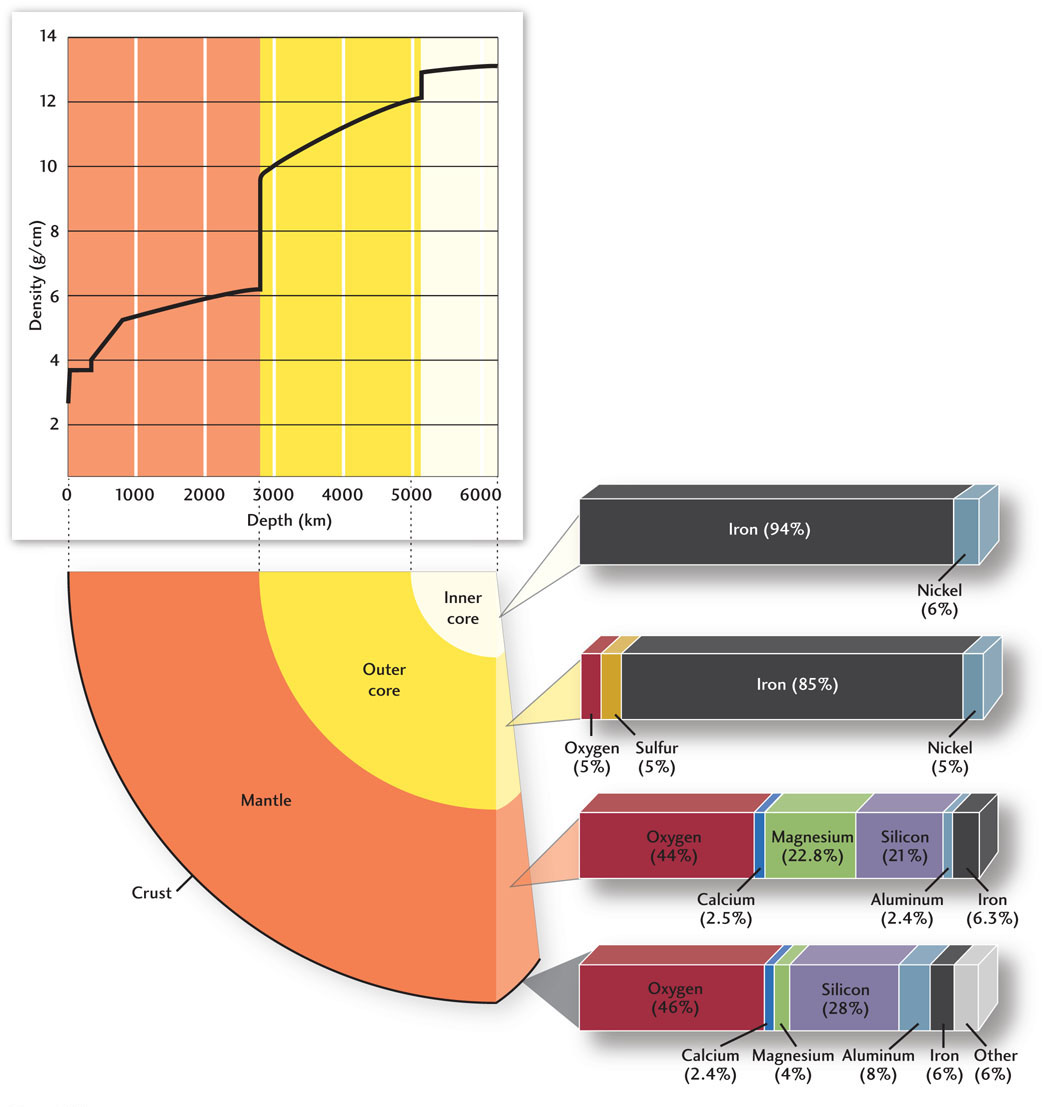
By the mid-twentieth century, geologists had discovered all of Earth’s major layers—crust, mantle, outer core, and inner core—plus a number of more subtle features in its interior. They found, for example, that the mantle itself is layered into an upper mantle and a lower mantle, separated by a transition zone where the rock density increases in a series of steps. These density steps are not caused by changes in the rock’s chemical composition, but rather by changes in the compactness of its constituent minerals due to the increasing pressure with depth. The two largest density jumps in the transition zone are located at depths of about 410 km and 660 km, but they are smaller than the density increases across the Moho and core-mantle boundaries, which are due to changes in chemical composition (Figure 1.12).
13
Geologists were also able to show that Earth’s outer core cannot be made of a pure iron-nickel alloy, because the densities of these metals are higher than the observed density of the outer core. About 10 percent of the outer core’s mass must be made of lighter elements, such as oxygen and sulfur. On the other hand, the density of the solid inner core is slightly higher than that of the outer core and is consistent with a nearly pure iron-nickel alloy.
By bringing together many lines of evidence, geologists have put together a model of the composition of Earth and its various layers. In addition to the seismic data, that evidence includes the compositions of crustal and mantle rocks as well as the compositions of meteorites, thought to be samples of the cosmic material from which planets like Earth were originally made.
Only eight elements, out of more than a hundred, make up 99 percent of Earth’s mass (see Figure 1.12). In fact, about 90 percent of Earth consists of only four elements: iron, oxygen, silicon, and magnesium. The first two are the most abundant elements, each accounting for nearly a third of the planet’s overall mass, but they are distributed very differently. Iron, the densest of these common elements, is concentrated in the core, whereas oxygen, the least dense, is concentrated in the crust and mantle. The crust contains more silica than the mantle, and the core almost none. These relationships confirm Wiechert’s hypothesis that the different compositions of Earth’s layers are primarily the work of gravity. As you can see in Figure 1.12, the crustal rocks on which we stand are almost 50 percent oxygen!