Fossil-Fuel Resources
Economically valuable deposits of our most important energy sources, fossil fuels, develop under special environmental and geologic conditions. Fossil fuels come from the organic debris of former life: plants, algae, bacteria, and other microorganisms that have been buried, transformed, and preserved in sediments.
647
How Do Oil and Gas Form?
Oil and gas begin to form in sedimentary basins where the production of organic matter is high and the supply of oxygen in the sediments is inadequate to decompose all the organic matter they contain. Many offshore thermal subsidence basins on continental margins satisfy both these conditions. In such environments, and to a lesser degree in some river deltas and inland seas, the rate of sedimentation is high, and organic matter is buried and protected from decomposition.
During millions of years of burial, chemical reactions triggered by the elevated temperatures and pressures found deep in the sediments slowly transform some of the organic material in these source beds into combustible hydrocarbons. The simplest hydrocarbon is methane gas (CH4), the compound we call natural gas. Raw petroleum, or crude oil, includes a diverse class of liquids composed of more complex hydrocarbons.
Crude oil forms at a limited range of pressures and temperatures, known as the oil window, usually found at depths between about 2 and 5 km (see the Practicing Geology Exercise in Chapter 5). Above the oil window, temperatures are too low (generally below 50°C) for the maturation of organic material into hydrocarbons, whereas below the oil window, temperatures are so high (greater than 150°C) that the hydrocarbons that form are broken down into methane, producing only natural gas.
As burial progresses, compaction of the source beds forces crude oil and natural gas into adjacent beds of permeable rock (such as sandstones or porous limestones), which act as hydrocarbon reservoirs. The relatively low densities of oil and gas cause them to rise, so that they float atop the water that almost always occupies the pores of permeable rock formations.
Where Do We Find Oil and Gas?
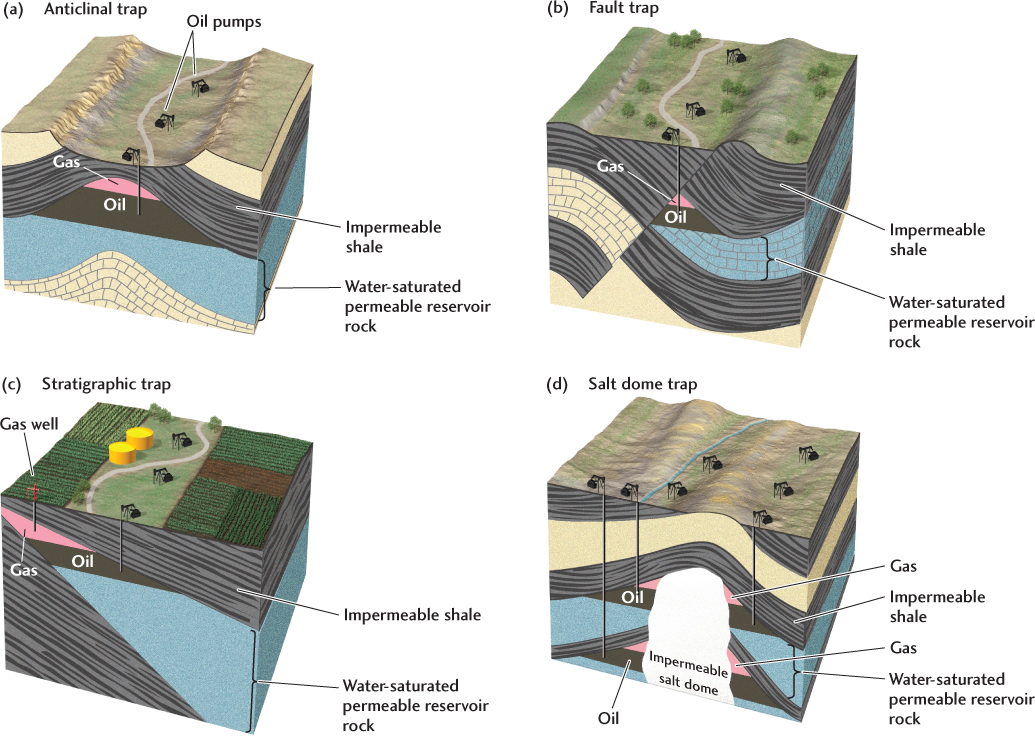
The conditions that favor large-scale accumulation of oil and natural gas are combinations of geologic structures and rock types that create an impermeable barrier to upward migration, forming an oil trap (Figure 23.8). Some oil traps, called structural traps, are created by deformation structures. One type of structural trap is formed by an anticline in which an impermeable layer of shale overlies a permeable sandstone formation (Figure 23.8a). The oil and gas accumulate at the crest of the anticline—the gas highest, the oil next—both floating on the groundwater that saturates the sandstone (see Chapter 7, Practicing Geology). Similarly, an angular unconformity or displacement at a fault may place a dipping permeable limestone formation opposite an impermeable shale, creating another type of structural trap (Figure 23.8b). Other types of oil traps are created by the original pattern of sedimentation, as when a dipping permeable sandstone formation thins out against an impermeable shale (Figure 23.8c). These structures are called stratigraphic traps. Oil can also be trapped against an impermeable mass of salt in a salt dome trap (Figure 23.8d).
648
The hydrocarbon reservoirs that hold oil and natural gas are complex geologic systems. Geologists can map the reservoir rocks in three dimensions using various techniques, such as seismic imaging (see Figure 14.6). The three-dimensional models they obtain show them where the bulk of the oil and gas is located and allow them to predict how it will flow from holes drilled into the reservoir.
In their search for petroleum resources, geologists have seismically mapped thousands of oil traps throughout the world. Only a fraction of them have proven to contain economically valuable amounts of oil or gas, because traps alone are not enough to create a hydrocarbon reservoir. A trap will contain oil only if source beds were present, the necessary chemical reactions took place, and the oil migrated into the trap and stayed there without being disturbed by subsequent heating or deformation. Although oil and gas are not rare, most of the large, easy-to-find deposits have already been located, and the discovery of new resources is becoming more difficult.
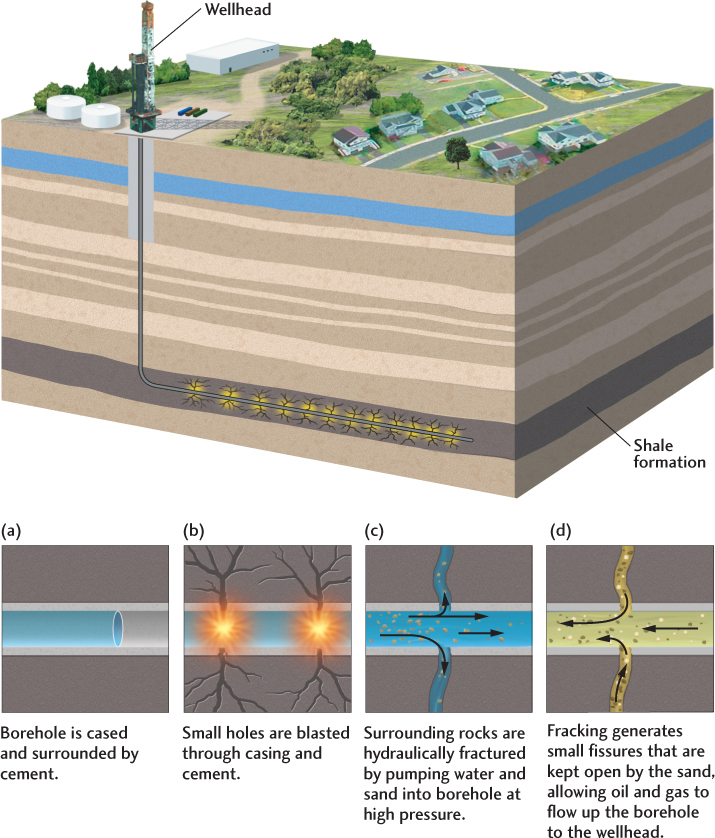
Efforts are now under way to find more efficient ways to extract oil and natural gas from deep rock formations. Drilling holes deep into Earth’s crust has become a very sophisticated and expensive business (Figure 23.9). Petroleum engineers use three-dimensional models to steer drill bits on swooping paths into the richest parts of a reservoir. They inject water into stubborn formations to coax the oil out, a processes known as hydraulic fracturing (“fracking”), and they pump carbon dioxide down strategically positioned drill holes to push the oil into areas where it can be more efficiently recovered through other drill holes. These methods have increased the fraction of oil and gas that can be extracted, increasing reserves.

Distribution of Oil Reserves
In the decade 2002–2012, the world consumed about 0.3 trillion barrels of oil (1 barrel = 42 gallons); yet, the worldwide reserves of oil did not decline; in fact, they increased from about 1.3 trillion barrels to almost 1.7 trillion barrels. Oil exploration is an immensely successful geologic activity!
Oil reserves and their decadal changes are displayed by region in Figure 23.10. The oil fields of the Middle East—including Iran, Kuwait, Saudi Arabia, Iraq, and the Baku region of Azerbaijan—account for 48 percent of the world’s total. Here, sediments rich in organic material have been folded and faulted by the closure of the ancient Tethys Ocean, forming a nearly ideal environment for oil accumulation. The extensive reservoirs discovered in this vast convergence zone include the world’s largest, the Ghawar field in Saudi Arabia. Ghawar has produced more than 70 billion barrels of oil since its opening in 1948 and may produce another 70 billion barrels over its remaining lifetime.
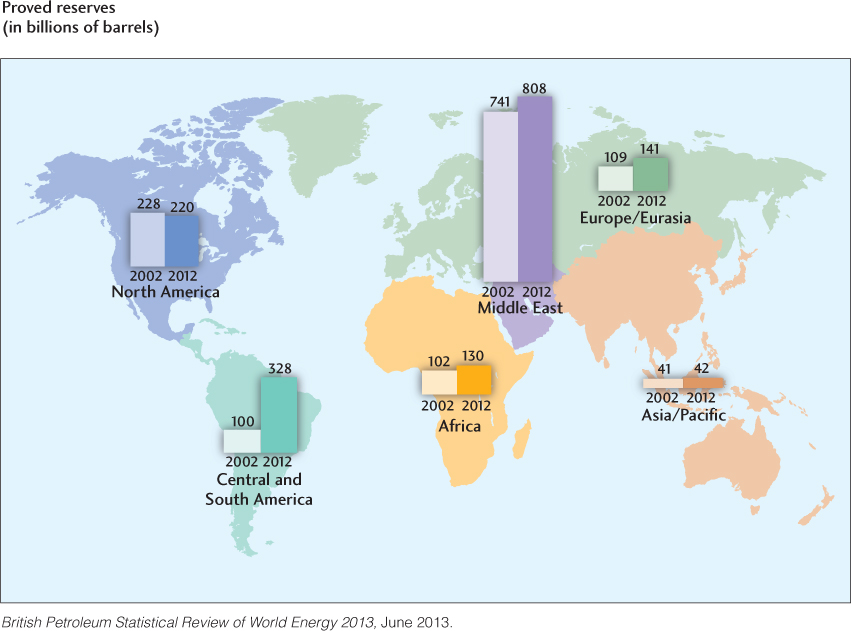
Most of the oil reserves in the Western Hemisphere are located in the highly productive Gulf Coast–Caribbean area, which includes the Louisiana-Texas region, Mexico, Colombia, and Venezuela. The threefold increase in South American oil reserves from 2002 to 2012 (see Figure 23.10) came mainly from improvements in oil-recovery technology, which will allow the heavy oil of Venezuela’s Orinoco Basin to be exploited economically, as well as the discovery of huge new oil fields in the Atlantic Ocean off Brazil.
The United States’ oil reserves also increased, from 31 billion barrels in 2002 to 35 billion barrels in 2012, placing it tenth worldwide. Thirty-one U.S. states have commercial oil reserves, and small, noncommercial resources can be found in most of the others.
649
Oil Production and Consumption
Global oil production in 2012 was about 31 billion barrels per year worldwide. The United States produced 3.2 billion barrels, more than any other nation except Saudi Arabia and Russia, but it consumed about 6.8 billion barrels. This gap between U.S. production and consumption, about 3.5 billion barrels, must be filled by importing oil. This imbalance—$327 billion in 2011—has contributed more than any other factor to the massive U.S. foreign trade deficit.
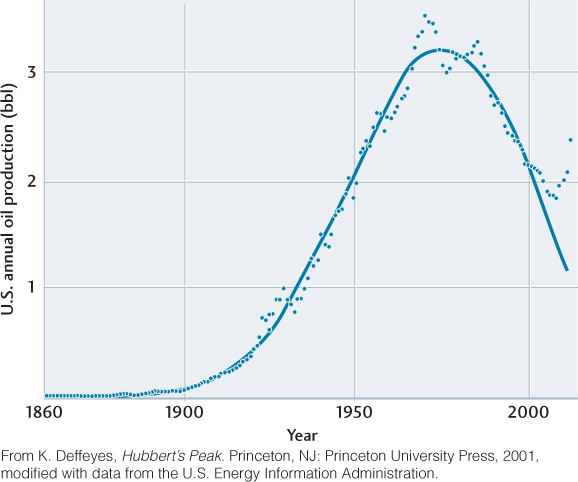
The United States is a “mature” oil producer, in the sense that most of the petroleum reserves within its borders have already been exploited. Production reached a maximum in 1970 and declined, approximately following a bell-shaped curve (Figure 23.11). The high point of the curve is referred to as Hubbert’s peak, named for petroleum geologist M. King Hubbert. In 1956, Hubbert used a simple mathematical relationship between the production rate and the rate of discovery of new reserves to predict that U.S. oil production, which was growing rapidly at the time, would actually begin to decline sometime in the early 1970s. His arguments were roundly dismissed as overly pessimistic, but history proved him right: production did indeed peak in 1970, beginning a decline that continued much as Hubbert predicted throughout the late twentieth century.
650
However, in 2009, U.S. oil production began to increase again, with 2012 production 30 percent higher than its minimum in 2008. This increase signals a new U.S. oil boom, fed by the rapid development of offshore oil fields and improved technology for recovering oil on land, including the controversial technique of fracking.
When Will We Run Out of Oil?
At the current production rate, the world will consume all of its known oil reserves in about 55 years. Does that mean we will run out of oil before the end of this century? No, because oil resources are much greater than oil reserves.
In fact, we will never really “run out” of oil. As resources diminish, prices will eventually rise so high that we cannot afford to waste oil by burning it as a fuel. Its main use will then be as a raw material for producing plastics, fertilizers, and a host of other petrochemical products. The petrochemical industry is already a very big business, consuming 7 percent of global oil production. As oil geologist Ken Deffeyes has noted, future generations will probably look back on the Petroleum Age with a certain amount of disbelief: “They burned it? All those lovely organic molecules, and they just burned it?”
The key question is not when oil will run out, but when oil production will stop rising and begin to decline. This milestone—Hubbert’s peak for world oil production—is the real tipping point; once it is reached, the gap between supply and demand will grow rapidly, driving oil prices sky-high.
So how close are we to Hubbert’s peak? The answer to this question is the subject of considerable debate. Some oil pessimists have argued that we are fast approaching Hubbert’s peak. Oil optimists, on the other hand, believe that new oil discoveries and improvements in oil-recovery technologies, such as fracking and deep-water drilling, will provide enough petroleum to satisfy world demand for many decades into the future. The recent increases in oil reserves shown in Figure 23.10 support this view.
Oil and the Environment
Extracting fossil fuels can have a number of detrimental effects on the environment. On April 20, 2010, an explosion aboard the drilling platform Deepwater Horizon killed 11 men and injured 17 others. This blowout resulted in the largest marine oil spill in history, releasing 5 million barrels of crude oil into the Gulf of Mexico during the next 3 months (Figure 23.12). The oil spill caused significant environmental damage to ecosystems along the Gulf Coast (Figure 23.13).
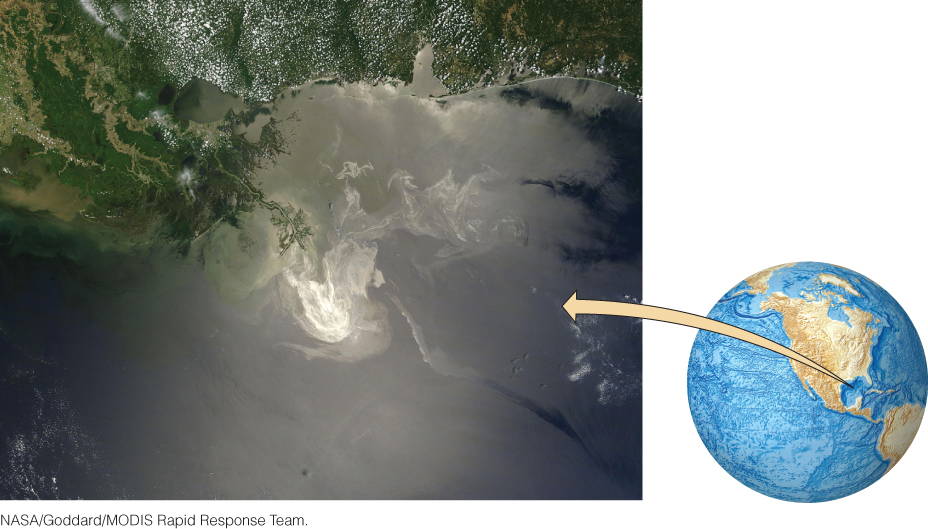

This accident, like earlier spills off the Yucatan coast in 1979 and Santa Barbara in 1969, renewed the long-running debate about whether to allow drilling for oil and natural gas in fragile habitats such as the Arctic National Wildlife Refuge (ANWR) on the coastal plain of northern Alaska (Figure 23.14). The total petroleum resource in ANWR has not been fully evaluated, but it could be as much as 40 billion barrels of oil. The USGS estimates that if oil prices were high enough, 6 billion to 16 billion barrels of this oil could be produced economically using current technologies. There is no doubt that these resources would contribute to the national economy. But oil and gas production would require the building of roads, pipelines, and housing in a delicate environment that is an important breeding area for caribou, musk-oxen, snow geese, and other wildlife. Policy makers must weigh the short-term economic benefits of drilling against possible long-term environmental losses in making this decision.

651
Natural Gas
The world’s reserves of natural gas are comparable to its crude oil reserves (see Figure 23.7) and will likely exceed them in the decades ahead. Estimates of natural gas resources have been rising in recent years because exploration for natural gas has increased, and gas reservoirs have been identified in new settings, such as very deep rock formations, overthrust structures, coal beds, tight (less permeable) sandstones, and shales.
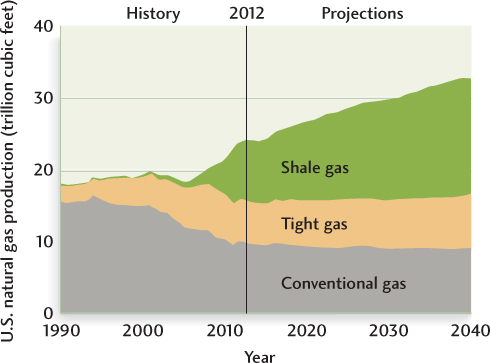
As in the case of oil, newer technologies have increased the efficiency of gas production and the types of rock formations from which gas can be extracted. In a method called hydraulic fracturing (or “fracking”), large amounts of water are injected through the drilling pipe into a hard rock formation, such as a shale, to create tiny fissures in the rock, which allows gas to flow more readily into the pipe (Figure 23.15). This technology has created a boom in the extraction of natural gas from shale formations such as the Marcellus shale that underlies the northern Appalachian Mountains and the Allegheny Plateau of the eastern United States (see Chapter 5). The production of “shale gas” has increased tenfold in the last decade and now accounts for almost one-third of U.S. natural gas production (Figure 23.16).
652
The environmental cost associated with fracking can be steep, however, because the process uses huge amounts of water, and wastes from shale gas production can contaminate the local water supply. Moreover, the disposal of waste water and chemicals used in fracking is often done by injecting these fluids into deep wells, which lubricates old faults in Earth’s crust, causing earthquakes (see Chapter 13, Practicing Geology). This practice has increased earthquake activity in many regions of the United States where the seismicity has been historically low, such as Oklahoma, Texas, and Ohio.
653
Natural gas is a premium fuel for a number of reasons. In combustion, methane combines with atmospheric oxygen, releasing energy in the form of heat and producing only carbon dioxide and water. Natural gas therefore burns much more cleanly than oil or coal, which also produces sulfur dioxide (the major cause of acid rain). Moreover, natural gas emits 30 percent less CO2 per unit of energy than oil and more than 40 percent less than coal. Therefore, substituting natural gas for coal as, say, the fuel for power plants lowers the carbon intensity of energy production—that is, carbon emissions per quad of electricity. Natural gas is easily transported across continents through pipelines. Getting it from source to market across oceans has been more difficult. The construction of tankers and ports that can handle liquefied natural gas (LNG) is beginning to solve this problem, although the potential dangers (such as the risk of a large explosion) have made LNG facilities controversial in the communities where they would be located.
Natural gas accounts for about 33 percent of all fossil-fuel consumption in the United States each year (see Figure 23.5). More than half of U.S. homes and a great majority of commercial and industrial buildings are connected to a network of underground pipelines that draw gas from fields in the United States, Canada, and Mexico. The rise of natural gas resources has led some observers to speculate that we are now transitioning from a “petroleum economy” to a “methane economy.”
Coal
The abundant plant fossils found in coal beds show that coal is a biological sediment formed from large accumulations of plant material in wetlands. As the luxuriant plant growth of a wetland dies, leaves, twigs, and branches fall to the waterlogged soil. Rapid burial and immersion in water protect this plant material from complete decay because the bacteria that decompose organic matter are cut off from the oxygen they need. The plant material accumulates and gradually turns into peat, a porous brown mass of organic matter in which twigs, roots, and other plant parts can still be recognized (Figure 23.17). The accumulation of peat in oxygen-poor environments can be seen in modern swamps and peat bogs. When dried, peat burns readily because it is 50 percent carbon.
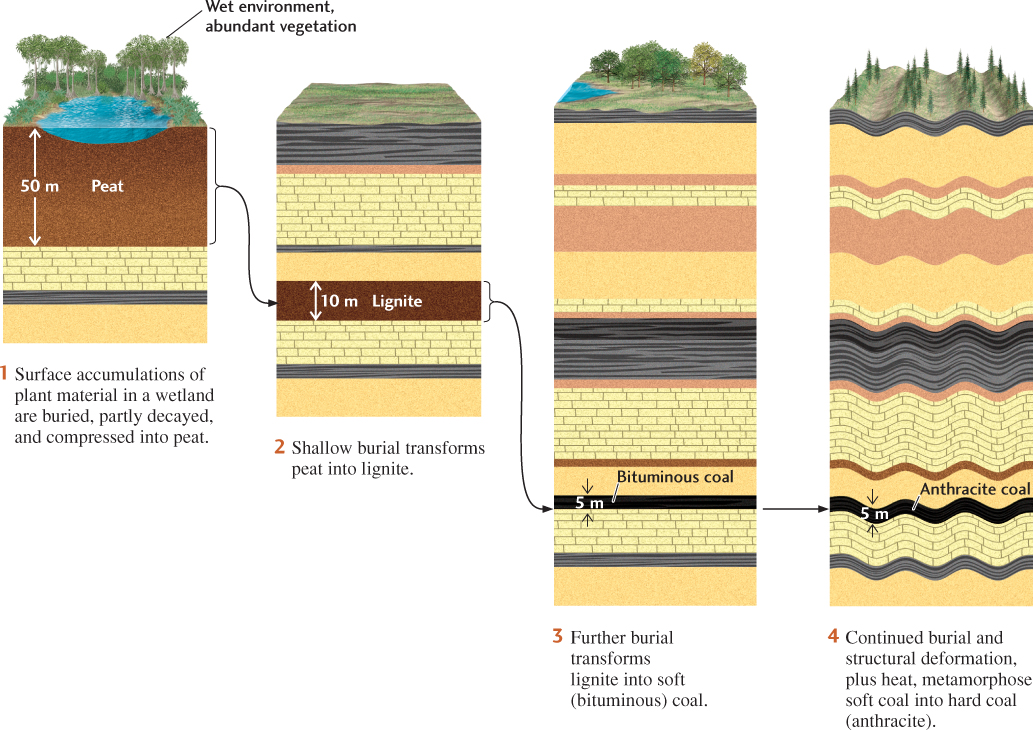
Over time, with continued burial, the peat is compressed and heated. Chemical transformations increase the peat’s already high carbon content, and it becomes lignite, a very soft, brownish black, coal-like material containing about 70 percent carbon. The higher temperatures and deformation that accompany greater depths of burial may transform lignite into subbituminous and bituminous coal, or soft coal, and ultimately into anthracite, or hard coal. The higher the grade of metamorphism, the harder and more vitreous the coal, and the higher its carbon content, and therefore its energy content. Anthracite is more than 90 percent carbon.
654
Coal Resources
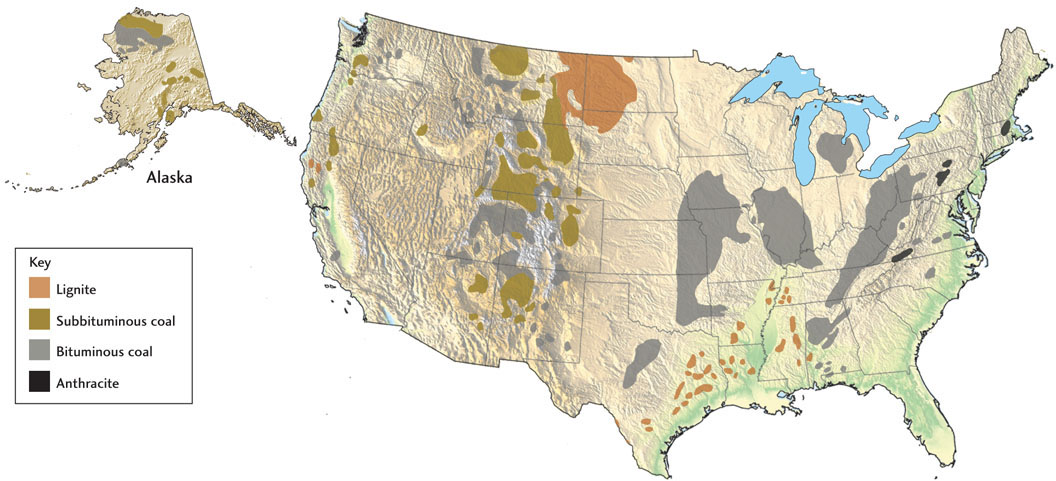
There are huge resources of coal in sedimentary rocks. Although coal has been a major energy source since the late nineteenth century, only about a few percent of the world’s coal reserves have been consumed. According to the best estimates, these reserves amount to 860 billion metric tons, which are capable of producing 17,800 quads of energy, more than any other fossil fuel (see Figure 23.7). About 85 percent of the world’s coal resources are concentrated in the former Soviet Union, China, and the United States; these areas are also the world’s largest coal producers. The United States has extensive deposits of coal in many states (Figure 23.18)—enough to last for a few hundred years at the nation’s current rate of use (about a billion tons per year). From 1975, when the price of oil began its precipitous rise, until 2005, coal supplied an increasing proportion of U.S. energy needs, primarily as fuel for electrical power generation. But coal usage has since declined as natural gas production has increased (see Figure 23.3). Coal currently accounts for about 18 percent of U.S. energy consumption.
The Costs of Coal
The extraction and combustion of coal present serious problems that make it a less desirable fuel than oil or natural gas. Underground coal mining is a dangerous profession; more than 2000 miners are killed each year in China alone. Many more coal miners suffer from black lung, a debilitating inflammation of the lungs caused by the inhalation of coal particles. Surface or “strip” mining—the removal of soil and surface sediments to expose coal beds—is safer for the miners, but it can ravage the countryside if the land is not restored. An especially destructive type of surface mining, now common in the Appalachian Mountains of the eastern United States, is “mountaintop removal,” in which up to 300 vertical meters of a mountain crest is blasted away to expose underlying coal beds (Figure 23.19). The excess rock and soil are dumped into the surrounding valleys.

Coal is a notoriously dirty fuel. When burned, it produces, on average, 25 percent more CO2 per unit of energy than oil and 70 percent more than natural gas; in other words, its carbon intensity is high. Most coal also contains appreciable amounts of pyrite, which is released into the atmosphere as noxious sulfur-containing gases when the coal is burned. Acid rain, which forms when these gases combine with rainwater, has been a severe problem in Canada, Scandinavia, and the northeastern United States, and eastern Europe.
An inorganic residue, called coal ash, remains after coal is burned. Coal ash contains all the metals that were present in the coal, some of which, such as mercury, are toxic. Coal ash can amount to several tons for every 100 tons of coal burned, so it poses a significant disposal problem. Ash can also escape from smokestacks, creating a health risk to people downwind.
U.S. government regulations now require industries that burn coal to adopt technologies for “clean” coal combustion, which have reduced emissions of sulfur and toxic chemicals. Federal laws also mandate the restoration of land disrupted by surface mining and the reduction of dangers to miners. These measures are expensive and add to the cost of coal, but it is still a relatively inexpensive fuel compared with petroleum.
655
Unconventional Hydrocarbon Resources
Extensive deposits of hydrocarbons occur in two other forms: source beds that are rich in organic material but never reached the oil window, and formations that once contained oil but have since “dried out,” losing many of their volatile components, to form heavy oil or a tarlike substance called natural bitumen (not to be confused with bituminous coal).
A hydrocarbon resource of the first type is oil shale, a fine-grained, clay-rich sedimentary rock containing large amounts of organic matter. In the 1970s, oil producers began trying to commercialize the extensive oil shales of western Colorado and eastern Utah, but those efforts were largely abandoned by the 1980s as oil prices fell, concerns over environmental damage increased, and technical problems persisted. New oil-recovery technologies such as hydraulic fracturing has made energy production from oil shales much more efficient, but the environmental costs per unit of energy remain high. As we have previously noted, the process of extracting oil and gas from shales by fracking requires huge amounts of water, a scarce resource in the western United States, and the reinjection of the waste water into the crust can cause earthquakes, even in seismically inactive areas.
One deposit of the second type, the tar sands of Alberta, Canada, is estimated to contain a hydrocarbon reserve equivalent to 170 billion barrels of oil and a total resource perhaps 10 times that amount. More than 600 million barrels of oil are now extracted from the Alberta tar sands each year, and Canadian production is projected to increase fivefold by 2030, providing as much as 5 percent of world demand for fossil fuels. Development of the tar sands, like that of oil shales, raises important environmental concerns, however. It takes 2 tons of mined sand to produce 1 barrel of oil, leaving lots of waste sand, which is an environmental pollutant. Moreover, production of oil from the tar sands is an inefficient process that sucks up about two-thirds of the energy they ultimately render and emits considerably more CO2 than conventional oil production.