The Structure of Matter
In 1805, the English chemist John Dalton hypothesized that each of the various chemical elements consists of a different kind of atom, that all atoms of any given element are identical, and that chemical compounds are formed by various combinations of atoms of different elements in definite proportions. By the early twentieth century, physicists, chemists, and mineralogists, building on Dalton’s ideas, had come to understand the structure of matter much as we do today. We now know that an atom is the smallest unit of an element that retains the physical and chemical properties of that element. We also know that atoms are the small units of matter that combine in chemical reactions, and that atoms themselves are divisible into even smaller units.
The Structure of Atoms
Understanding the structure of atoms allows us to predict how chemical elements will react with one another and form new crystal structures. The structure of an atom is defined by a nucleus, which contains protons and neutrons and which is surrounded by electrons. (For a more detailed review of the structure of atoms, see Appendix 3.)
The Nucleus: Protons and Neutrons
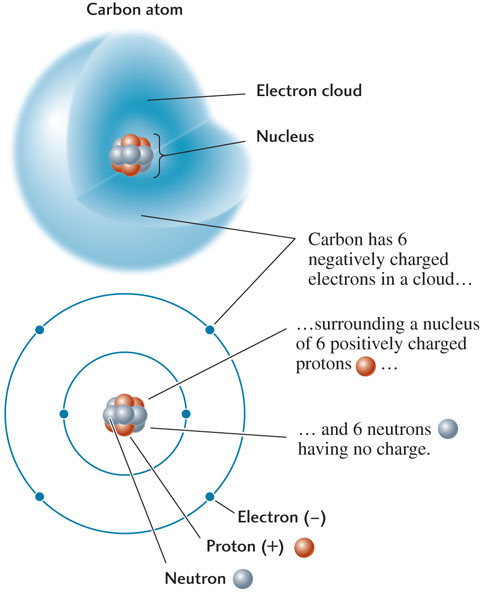
At the center of every atom is a dense nucleus containing virtually all the mass of the atom in two kinds of particles: protons and neutrons (Figure 3.2). A proton has a positive electrical charge of +1. A neutron is electrically neutral—that is, uncharged. Atoms of the same chemical element may have different numbers of neutrons, but the number of protons does not vary. For instance, all carbon atoms have six protons.
Electrons
Surrounding the nucleus is a cloud of moving particles called electrons, each with a mass so small that it is conventionally taken to be zero. Each electron carries a negative electrical charge of −1. The number of protons in the nucleus of any atom is balanced by the same number of electrons in the cloud surrounding the nucleus, so that the atom is electrically neutral. Thus, the nucleus of a carbon atom is surrounded by six electrons (see Figure 3.2).
Atomic Number and Atomic Mass
The number of protons in the nucleus of an atom is its atomic number. Because all atoms of the same element have the same number of protons, they also have the same atomic number. All atoms with six protons, for example, are carbon atoms (atomic number 6). In fact, the atomic number of an element can tell us so much about that element’s behavior that the periodic table organizes elements according to their atomic number (see Appendix 3). Elements in the same vertical column of the periodic table, such as carbon and silicon, tend to have similar chemical properties.
The atomic mass of an element is the sum of the masses of its protons and its neutrons. (Electrons, because they have so little mass, are not included in this sum.) Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons, and therefore different atomic masses. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes of the element carbon, for example, all have six protons, but may have six, seven, or eight neutrons, giving atomic masses of 12, 13, and 14.
In nature, the chemical elements exist as mixtures of isotopes, so their average atomic masses are never whole numbers. The atomic mass of carbon, for example, is 12.011. It is close to 12 because the isotope carbon-12 is by far the most abundant. The relative abundances of the various isotopes of an element on Earth are determined by processes that enhance the abundances of some isotopes over others. Carbon-12, for example, is favored by some chemical reactions, such as photosynthesis, in which organic carbon compounds are produced from inorganic carbon compounds.
60
Chemical Reactions
The structure of an atom determines its chemical reactions with other atoms. Chemical reactions are interactions of the atoms of two or more chemical elements in certain fixed proportions that produce chemical compounds. For example, when two hydrogen atoms combine with one oxygen atom, they form a new chemical compound, water (H2O). The properties of a chemical compound may be entirely different from those of its constituent elements. For example, when an atom of sodium, a metal, combines with an atom of chlorine, a noxious gas, they form the chemical compound sodium chloride, better known as table salt. We represent this compound by the chemical formula NaCl, in which the symbol Na stands for the element sodium and the symbol Cl for the element chlorine. (Every chemical element has been assigned its own symbol, which we use in a kind of shorthand for writing chemical formulas and equations; these symbols are given in the periodic table in Appendix 3.)
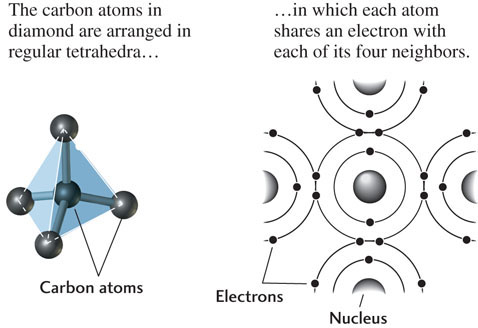
Chemical compounds, such as minerals, are formed either by electron sharing between the reacting atoms or by electron transfer between the reacting atoms. Carbon and silicon, two of the most abundant elements in Earth’s crust, tend to form compounds by electron sharing. Diamond is a compound composed entirely of carbon atoms sharing electrons (Figure 3.3).
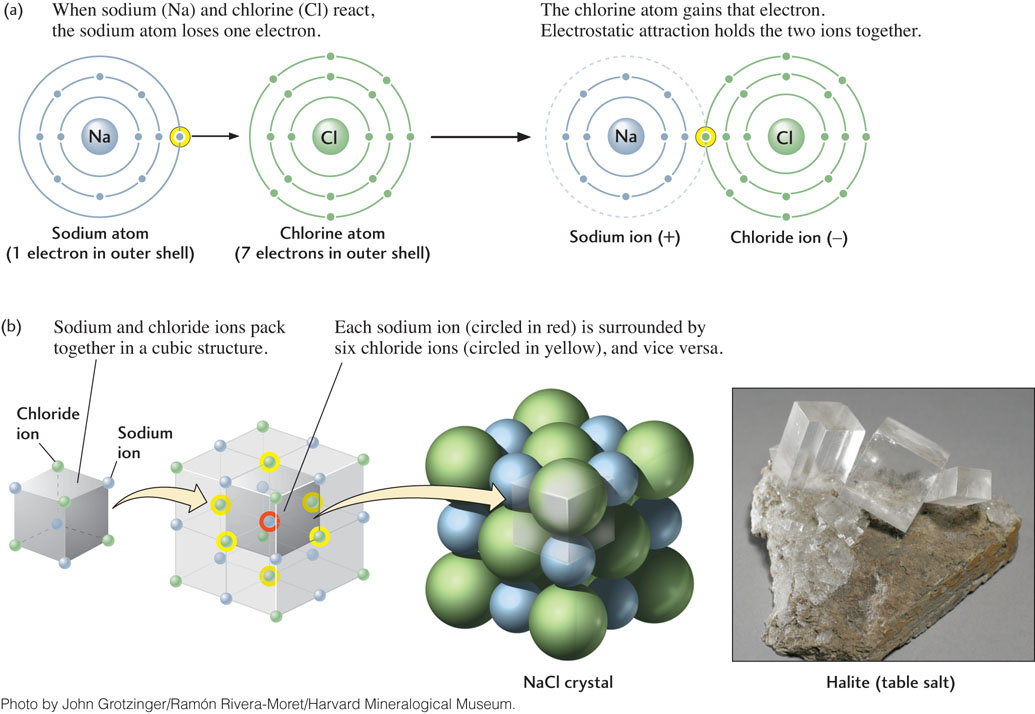
In the reaction between sodium (Na) and chlorine (Cl) atoms to form sodium chloride (NaCl), electrons are transferred. The sodium atom loses one electron, which the chlorine atom gains (Figure 3.4a). An atom or group of atoms that has an electrical charge, either positive or negative, because of the loss or gain of one or more electrons is called an ion. Because the chlorine atom has gained a negatively charged electron, it is now a negatively charged ion, Cl−. Likewise, the loss of an electron gives the sodium atom a positive charge, making it a sodium ion, Na+. The compound NaCl itself remains electrically neutral because the positive charge on Na+ is exactly balanced by the negative charge on Cl−. A positively charged ion is called a cation, and a negatively charged ion is called an anion.
61
Chemical Bonds
When a chemical compound is formed either by electron sharing or by electron transfer, the ions or atoms that make up the compound are held together by electrostatic attraction between negatively charged electrons and positively charged protons. The attractions, or chemical bonds, between shared electrons or between gained and lost electrons may be strong or weak. Strong bonds keep a substance from decomposing into its elements or into other compounds. They also make minerals hard and keep them from cracking or splitting. Two major types of bonds are found in most rock-forming minerals: ionic bonds and covalent bonds.
Ionic Bonds
The simplest form of chemical bond is the ionic bond. Bonds of this type form by electrostatic attraction between ions of opposite charge, such as Na+ and Cl− in sodium chloride (see Figure 3.4a), when electrons are transferred. This attraction is of exactly the same nature as the static electricity that can make nylon or silk clothing cling to the body. The strength of an ionic bond decreases greatly as the distance between ions increases, and it increases as the electrical charges of the ions increase. Ionic bonds are the dominant type of chemical bonds in mineral structures: about 90 percent of all minerals are essentially ionic compounds.
Covalent Bonds
Elements that do not readily gain or lose electrons to form ions, and instead form compounds by sharing electrons, are held together by covalent bonds. These bonds are generally stronger than ionic bonds. One mineral with a covalently bonded crystal structure is diamond, which consists of a single element, carbon. Each carbon atom can share four of its own electrons with other carbon atoms and can acquire another four electrons by sharing with other carbon atoms. In diamond, every carbon atom is surrounded by four others arranged in a four-sided pyramidal form (a tetrahedron), each side of which is a triangle (see Figure 3.3). In this configuration, each carbon atom shares an electron with each of its four neighbors, resulting in a very stable configuration. (Figure 3.10 shows a network of carbon tetrahedra linked together.)
Metallic Bonds
Atoms of metallic elements, which have strong tendencies to lose electrons, pack together as cations, and the freely mobile electrons are shared and dispersed among those cations. This free electron sharing results in a kind of covalent bond that we call a metallic bond. It is found in a small number of minerals, among them the metal copper and some sulfides.
The chemical bonds of some minerals are intermediate between pure ionic and pure covalent bonds because some electrons are exchanged and others are shared.