Classes of Rock-Forming Minerals
All minerals on Earth have been grouped into seven classes according to their chemical composition (Table 3.1). Some minerals, such as copper, occur naturally as un-ionized pure elements; these minerals are classified as native elements. Most other minerals are classified by their anions. Olivine, for example, is classified as a silicate by its silicate anion, SiO44−. Halite (sodium chloride, NaCl) is classified as a halide by its chloride anion, Cl−. So is its close relative, sylvite (potassium chloride, KCl).
| Class | Defining Anions | Example |
|---|---|---|
| Native elements | None: no charged ions | Copper metal (Cu) |
| Oxides | Oxygen ion (O2−) | Hematite (Fe2O3) |
| Halides | Chloride (Cl−), fluoride (F−), | Halite (NaCl) |
| bromide (Br−), iodide (I−) | ||
| Carbonates | Carbonate ion (CO32−) | Calcite (CaCO3) |
| Sulfates | Sulfate ion (SO42−) | Anhydrite (CaSO4) |
| Silicates | Silicate ion (SiO44−) | Olivine (Mg,Fe)2SiO4 |
| Sulfides | Sulfide ion (S2−) | Pyrite (FeS2) |
65
Although many thousands of minerals are known, geologists commonly encounter only about 30 of them. These minerals are the building blocks of most crustal rocks and are called rock-forming minerals. Their relatively small number corresponds to the small number of elements that are abundant in Earth’s crust.
In the following pages, we consider the five most common classes of rock-forming minerals:
 Silicates, the most abundant class of minerals in Earth’s crust, are composed of oxygen (O) and silicon (Si)—the two most abundant elements in the crust—mostly in combination with cations of other elements.
Silicates, the most abundant class of minerals in Earth’s crust, are composed of oxygen (O) and silicon (Si)—the two most abundant elements in the crust—mostly in combination with cations of other elements. Carbonates are minerals composed of carbon and oxygen—in the form of the carbonate anion (CO32−)—in combination with calcium and magnesium. Calcite (calcium carbonate, CaCO3) is one such mineral.
Carbonates are minerals composed of carbon and oxygen—in the form of the carbonate anion (CO32−)—in combination with calcium and magnesium. Calcite (calcium carbonate, CaCO3) is one such mineral. Oxides are compounds of the oxygen anion (O2−) and metallic cations; an example is the mineral hematite (iron oxide, Fe2O3).
Oxides are compounds of the oxygen anion (O2−) and metallic cations; an example is the mineral hematite (iron oxide, Fe2O3). Sulfides are compounds of the sulfide anion (S2−) and metallic cations; an example is the mineral pyrite (iron sulfide, FeS2).
Sulfides are compounds of the sulfide anion (S2−) and metallic cations; an example is the mineral pyrite (iron sulfide, FeS2). Sulfates are compounds of the sulfate anion (SO42−) and metallic cations; an example is the mineral anhydrite (calcium sulfate, CaSO4).
Sulfates are compounds of the sulfate anion (SO42−) and metallic cations; an example is the mineral anhydrite (calcium sulfate, CaSO4).
The other three chemical classes of minerals—native elements, hydroxides, and halides—are less common as rock-forming minerals.
Silicates
The basic building block of all silicate mineral structures is the silicate ion. It is a tetrahedron composed of a central silicon ion (Si4+) surrounded by four oxygen ions (O2−), and thus has the formula SiO44− (Figure 3.11). Because the silicate ion has a negative charge, it often bonds to cations to form minerals. The cations it typically bonds to include sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), and iron (Fe2+). Alternatively, the silicate ion can share oxygen ions with other silicate tetrahedra. Silicate tetrahedra can form a number of crystal structures: they may be isolated (linked only to cations), or they may be linked to other silicate tetrahedra in rings, single chains, double chains, sheets, or frameworks. Some of these structures are shown in Figure 3.11.
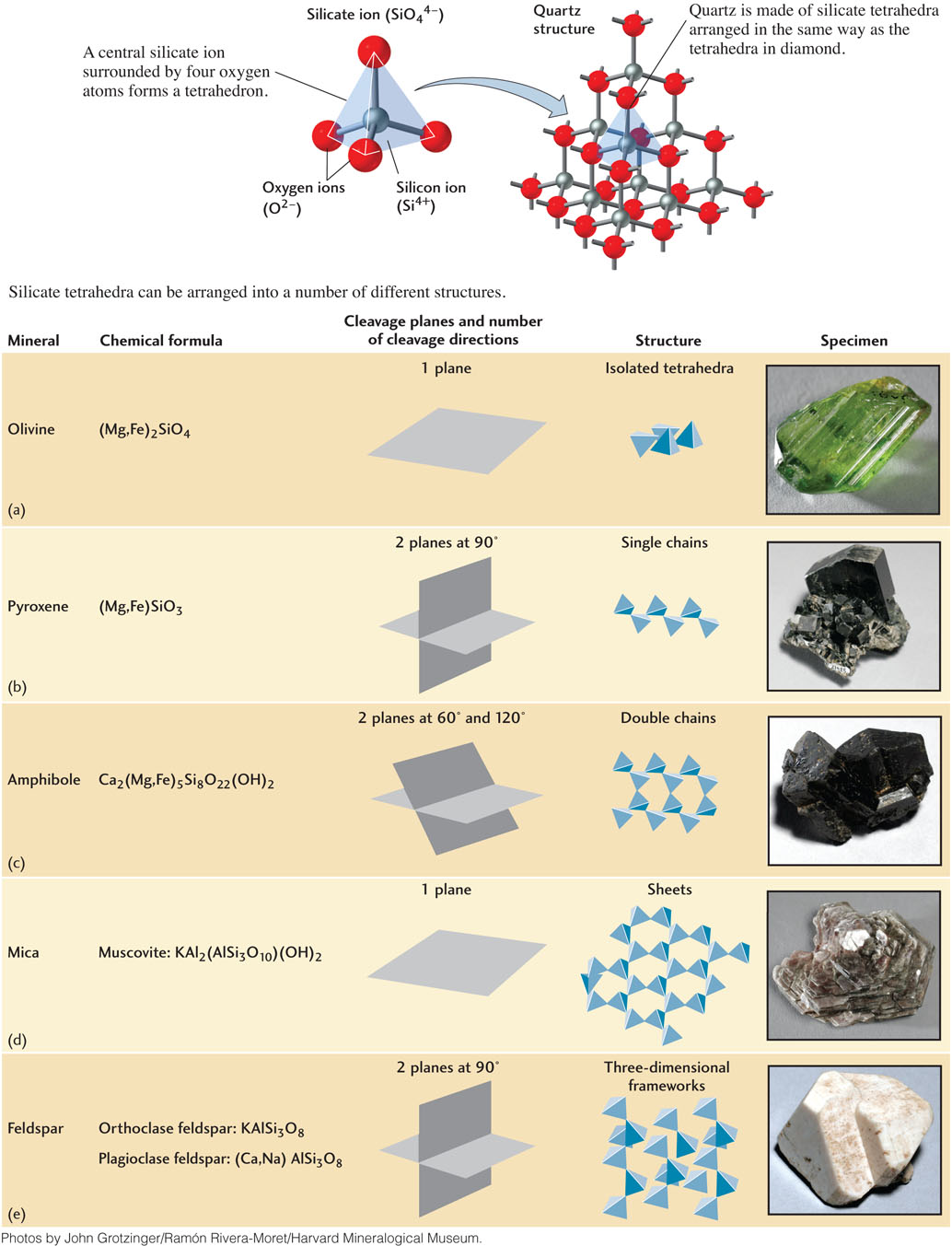
Isolated Tetrahedra
Isolated tetrahedra are linked by the bonding of each oxygen ion of the tetrahedron to a cation (Figure 3.11a). The cations, in turn, bond to the oxygen ions of other tetrahedra. The tetrahedra are thus isolated from one another by cations on all sides. Olivine is a rock-forming mineral with this structure.
Single-Chain Structures
Single chains are formed by the sharing of oxygen ions. Two oxygen ions of each silicate tetrahedron bond to adjacent tetrahedra in an open-ended chain (Figure 3.11b). These single chains are linked to other chains by cations. Minerals of the pyroxene group are single-chain silicate minerals. Enstatite, a pyroxene, contains iron or magnesium ions, or both; the two cations may substitute for each other, as in olivine. The formula (Mg,Fe)SiO3 represents this structure.
Double-Chain Structures
Two single chains may combine to form double chains linked to each other by shared oxygen ions (Figure 3.11c). Adjacent double chains linked by cations form the structure of minerals in the amphibole group. Hornblende, a member of this group, is an extremely common mineral in both igneous and metamorphic rocks. It has a complex composition that includes calcium (Ca2+), sodium (Na+), magnesium (Mg2+), iron (Fe2+), and aluminum (Al3+).
66
67
Sheet Structures
In sheet structures, each tetrahedron shares three of its oxygen ions with adjacent tetrahedra to build stacked sheets of tetrahedra (Figure 3.11d). Cations may be interlayered with the tetrahedral sheets. The micas and clay minerals are the most abundant sheet silicates. Muscovite, KAl2(AlSi3O10)(OH)2, is one of the most common sheet silicates and is found in many types of rocks. It can be separated into extremely thin, transparent sheets. Kaolinite, Al2Si2O5(OH)4, which also has this structure, is a common clay mineral found in sediments and is the basic raw material for pottery.
Frameworks
Three-dimensional frameworks may form as each tetrahedron shares all its oxygen ions with other tetrahedra. Feldspars, the most abundant minerals in Earth’s crust, are framework silicates (Figure 3.11e), as is another of the most common minerals, quartz (SiO2).
Silicate Compositions
Chemically, the simplest silicate is silicon dioxide, also called silica (SiO2), which is found most often as the mineral quartz. The silicate tetrahedra of quartz are linked, sharing two oxygen ions for each silicon ion, so the total formula adds up to SiO2.
In other silicate minerals, as we have seen, the basic structural units—rings, chains, sheets, and frameworks—are bonded to cations such as sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), and iron (Fe2+). As noted in the discussion of cation substitution, aluminum (Al3+) substitutes for silicon in many silicate minerals.
Carbonates
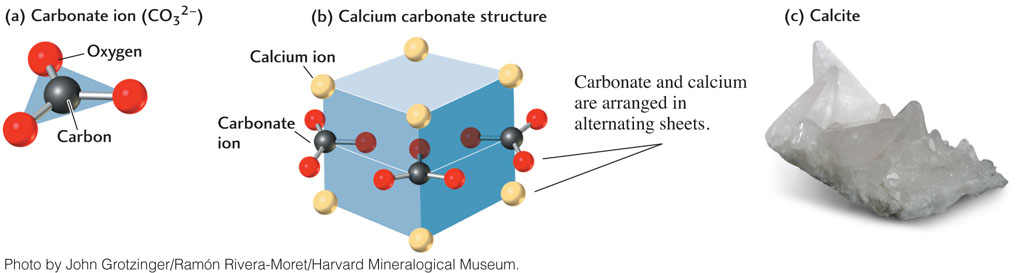
The basic building block of carbonate minerals is the carbonate ion (CO32−), which consists of a carbon ion surrounded by, and covalently bonded to, three oxygen ions in a triangle (Figure 3.12a). Groups of carbonate ions are arranged in sheets somewhat like those of the sheet silicates, which are linked together by layers of cations. In calcite (calcium carbonate, CaCO3), the sheets of carbonate ions are separated by layers of calcium ions (Figure 3.12b). Calcite is one of the most abundant minerals in Earth’s crust and is the chief constituent of a group of rocks called limestones (Figure 3.12c). Dolomite (CaMg(CO3)2) is another major mineral of crustal rocks that is made up of the same carbonate sheets separated by alternating layers of calcium ions and magnesium ions.
Oxides
Oxide minerals are compounds in which oxygen is bonded to atoms or cations of other elements, usually metallic cations such as iron (Fe2+ or Fe3+). Most oxide minerals are ionically bonded, and their structures vary with the size of the metallic cations. This class of minerals has great economic importance because it includes the ores containing many of the metals, such as chromium and titanium, used in the manufacture of metallic materials and devices. Hematite (Fe2O3) (Figure 3.13a) is a chief ore of iron.

Another group of abundant minerals in this class, the spinels (Figure 3.13b), are oxides of two metals, magnesium and aluminum (MgAl2O4). Spinels have a closely packed cubic structure and a high density (3.6 g/cm3), reflecting the conditions of high pressure and temperature under which they form. Transparent gem-quality spinels may resemble ruby or sapphire and are found in the crown jewels of England and Russia.
Sulfides
The chief ores of other valuable minerals—such as copper, zinc, and nickel—are members of the sulfide class. The basic building block of this class is the sulfide ion (S2−), a sulfur atom that has gained two electrons. In the sulfide minerals, the sulfide ion is bonded to metallic cations. Most sulfide minerals look like metals, and almost all are opaque. The most common sulfide mineral is pyrite (FeS2), often called “fool’s gold” because of its yellowish metallic appearance (Figure 3.14).

68
Sulfates
The basic building block of sulfates is the sulfate ion (SO42−). It is a tetrahedron made up of a central sulfur atom surrounded by four oxygen ions (O2−). One of the most abundant minerals of this class is gypsum (Figure 3.15), the primary component of plaster. Gypsum, a calcium sulfate, forms when seawater evaporates. During evaporation, Ca2+ and SO42−, two ions that are abundant in seawater, combine and precipitate as layers of sediment, forming calcium sulfate (CaSO4 • 2H2O). (The dot in this formula signifies that two water molecules are bonded to the calcium and sulfate ions.)

Another calcium sulfate, anhydrite (CaSO4), differs from gypsum in that it contains no water. (Its name is derived from the word anhydrous, meaning “free from water.”) Gypsum is stable at the low temperatures and pressures found at Earth’s surface, whereas anhydrite is stable at the higher temperatures and pressures where sedimentary rocks are buried.
As scientists discovered in 2004, sulfate minerals precipitated from water and formed sedimentary layers early in the history of Mars. These minerals were precipitated by processes similar to those observed on Earth when lakes and shallow seas dried up. Many of these sulfate minerals, however, are quite different from the sulfate minerals commonly found on Earth and include strange iron-bearing sulfates that precipitated from very harsh, acidic waters (see Earth Issues 11.1).
69