Physical Properties of Minerals
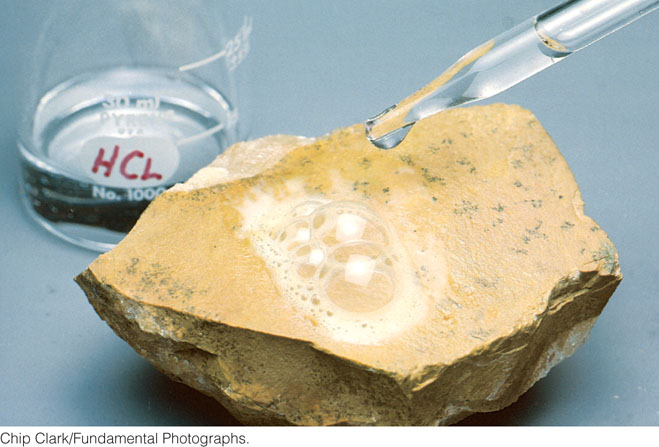
Geologists use their knowledge of mineral composition and structure to understand the origins of rocks. First they must identify the minerals that make up a rock. To do so, they rely greatly on chemical and physical properties that can be observed relatively easily. In the nineteenth and early twentieth centuries, geologists carried field kits for rough chemical analyses of minerals that would help in their identification. One such test is the origin of the phrase “the acid test.” It consists of dropping diluted hydrochloric acid (HCl) on a mineral to see if it fizzes (Figure 3.16). Fizzing indicates that carbon dioxide (CO2) is escaping, which means that the mineral is likely to be calcite, a carbonate.
In this section, we review the physical properties of minerals, many of which contribute to their practical and decorative value.
Hardness
Hardness is a measure of the ease with which the surface of a mineral can be scratched. Just as diamond, the hardest mineral known, scratches glass, a quartz crystal, which is harder than feldspar, scratches a feldspar crystal. In 1822, Friedrich Mohs, an Austrian mineralogist, devised a scale (now known as the Mohs scale of hardness) based on the ability of one mineral to scratch another. At one extreme is the softest mineral (talc); at the other, the hardest (diamond) (Table 3.2). The Mohs scale is still one of the best practical tools for identifying an unknown mineral. With a knife blade and a few of the minerals on the hardness scale, a field geologist can gauge an unknown mineral’s position on the scale. If the unknown mineral is scratched by a piece of quartz but not by the knife, for example, it lies between 5 and 7 on the scale.
| Mineral | Scale Number | Common Objects |
|---|---|---|
| Talc | 1 | |
| Gypsum | 2 | Fingernail |
| Calcite | 3 | Copper coin |
| Fluorite | 4 | |
| Apatite | 5 | Knife blade |
| Orthoclase | 6 | Window glass |
| Quartz | 7 | Steel file |
| Topaz | 8 | |
| Corundum | 9 | |
| Diamond | 10 |
70
Recall that covalent bonds are generally stronger than ionic bonds. The hardness of any mineral depends on the strength of its chemical bonds: the stronger the bonds, the harder the mineral. Within the silicate class of minerals, hardness varies with crystal structure, from 1 in talc, a sheet silicate, to 8 in topaz, a silicate with isolated tetrahedra. Most silicates fall in the 5 to 7 range on the Mohs scale. Only sheet silicates are relatively soft, with hardnesses between 1 and 3.
Within groups of minerals that have similar crystal structures, hardness is related to other factors that also affect bond strength:
 Size: The smaller the atoms or ions, the smaller the distance between them and the greater the electrostatic attraction—and thus the stronger the bond.
Size: The smaller the atoms or ions, the smaller the distance between them and the greater the electrostatic attraction—and thus the stronger the bond. Charge: The larger the charge of ions, the greater the attraction between them, and thus the stronger the bond.
Charge: The larger the charge of ions, the greater the attraction between them, and thus the stronger the bond. Packing: The closer the packing of atoms or ions, the smaller the distance between them, and thus the stronger the bond.
Packing: The closer the packing of atoms or ions, the smaller the distance between them, and thus the stronger the bond.
Size is an especially important factor for most metallic oxides and for most sulfides of metals with high atomic numbers, such as gold, silver, copper, and lead. Minerals of these groups are soft, with hardnesses of less than 3, because their metallic cations are so large. Carbonates and sulfates, whose structures are not closely packed, are also soft, with hardnesses of less than 5.
Cleavage
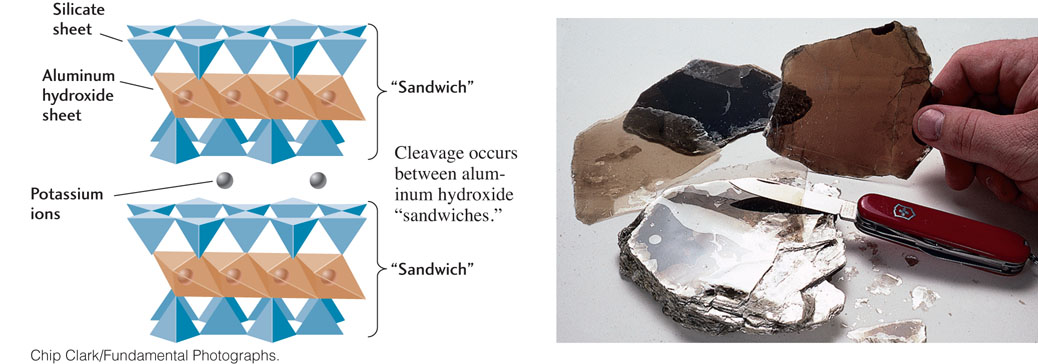
Cleavage is the tendency of a crystal to split along planar surfaces. The term cleavage is also used to describe the geometric pattern produced by such breakage. Cleavage varies inversely with bond strength: strong bonds produce poor cleavage, while weak bonds produce good cleavage. Because of their strength, covalent bonds generally produce poor or no cleavage. Ionic bonds are relatively weak, so they produce good cleavage. Even within a mineral that is entirely covalently bonded or entirely ionically bonded, however, bond strength varies along the different planes. For example, all of the bonds in diamond are covalent bonds, which are very strong, but some planes are more weakly bonded than others. Thus, diamond, the hardest mineral of all, can be cleaved along these weaker planes to produce perfect planar surfaces. Muscovite, a mica sheet silicate, splits along smooth, lustrous, flat, parallel surfaces, forming transparent sheets less than a millimeter thick. The excellent cleavage of micas results from the relative weakness of the bonds between its layers of cations sandwiched within sheets of silicate tetrahedra (Figure 3.17).
Cleavage is classified according to two primary sets of characteristics: the number of planes and pattern of cleavage, and the quality of surfaces and ease of cleaving.
Number of Planes and Pattern of Cleavage
The number of planes and pattern of cleavage are identifying hallmarks of many rock-forming minerals. Muscovite, for example, has only one plane of cleavage, whereas calcite and dolomite crystals have three cleavage planes that give them a rhomboidal shape (Figure 3.18).

A crystal’s structure determines its cleavage planes and its crystal faces. Crystals have fewer cleavage planes than possible crystal faces. Faces may be formed along any of numerous planes defined by rows of atoms or ions. Cleavage occurs along any of those planes across which the bonding is weak. All crystals of a mineral exhibit its characteristic cleavage planes, whereas only some crystals display particular faces.
71
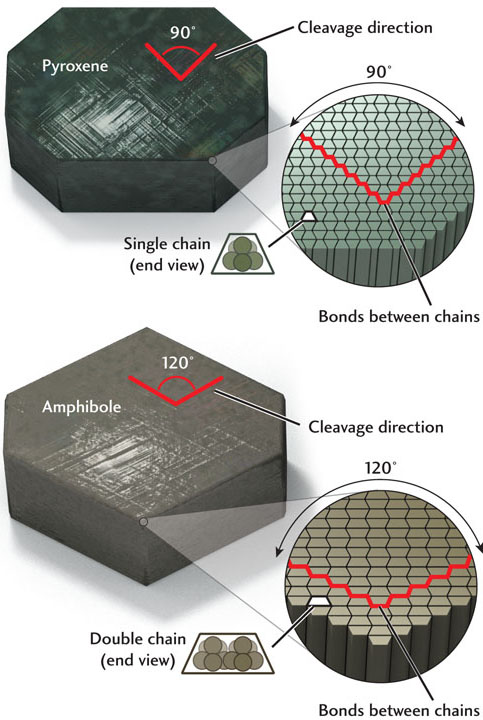
Distinctive angles of cleavage help identify two important groups of silicates, the pyroxenes and the amphiboles, that otherwise often look alike (Figure 3.19). Pyroxenes have a single-chain structure and are bonded so that their cleavage planes are almost at right angles (about 90°) to each other. In cross section, the cleavage pattern of pyroxenes is nearly a square. In contrast, amphiboles, which have a double-chain structure, are bonded so as to give two cleavage planes at about 60° and 120° to each other. They produce a diamond-shaped cross section.
Quality of Surfaces and Ease of Cleaving
A mineral’s cleavage is assessed as perfect, excellent, good, fair, poor, or none according to the quality of surfaces produced and the ease of cleaving. A few examples are described below.
Muscovite can be cleaved easily, and it produces extremely smooth surfaces; its cleavage is perfect. Single-chain and double-chain silicates (the pyroxenes and amphiboles, respectively) show good cleavage. Although these minerals split easily along the cleavage plane, they also break across it, producing cleavage surfaces that are not as smooth as those of micas. Fair cleavage is shown by the ring silicate beryl. Beryl’s cleavage is irregular, and the mineral breaks relatively easily along directions other than cleavage planes.
Many minerals are so strongly bonded that they lack even fair cleavage. Quartz, a framework silicate, is so strongly bonded in all directions that it breaks only along irregular surfaces. Garnet, a silicate with isolated tetrahedra, is also bonded strongly in all directions and so shows no cleavage. This absence of a tendency to cleave is found in most framework and isolated tetrahedral silicates.
Fracture
Fracture is the tendency of a crystal to break along irregular surfaces other than cleavage planes. All minerals show fracture, either across cleavage planes or—in such minerals as quartz—with no cleavage in any direction. Fracture is related to how bond strengths are distributed in directions that cut across cleavage planes. Fractures may be conchoidal, showing smooth, curved surfaces like those of a thick piece of broken glass. Another common fracture surface with an appearance like split wood is described as fibrous or splintery. The shapes and appearances of fracture surfaces depend on the particular structure and composition of the mineral.
Luster
The way the surface of a mineral reflects light gives it a characteristic luster. Mineral lusters are described by the terms listed in Table 3.3. Luster is controlled by the kinds of atoms present and their bonding, both of which affect the way light passes through or is reflected by the mineral. Ionically bonded crystals tend to have a glassy, or vitreous, luster, but covalently bonded materials are more variable. Many have an adamantine luster, like that of diamond. Pure metals, such as gold, and many sulfides, such as galena (lead sulfide, PbS) have a metallic luster. A pearly luster results from multiple reflections of light from planes beneath the surfaces of translucent minerals, such as the mother-of-pearl inner surfaces of many clamshells, which are made of the mineral aragonite. Luster, although an important criterion for field classification, depends heavily on the visual perception of reflected light. Textbook descriptions fall short of the actual experience of holding the mineral in your hand.
| Luster | Characteristics |
|---|---|
| Metallic | Strong reflections produced by opaque substances |
| Vitreous | Bright, as in glass |
| Resinous | Characteristic of resins, such as amber |
| Greasy | The appearance of being coated with an oily substance |
| Pearly | The whitish iridescence of such materials as pearl |
| Silky | The sheen of fibrous materials such as silk |
| Adamantine | The brilliant luster of diamond and similar minerals |
72
Color
The color of a mineral is imparted by light, either transmitted through or reflected by crystals or irregular masses of the mineral. The color of a mineral may be distinctive, but it is not the most reliable clue to its identity. Some minerals always show the same color; others may have a range of colors. Many minerals show a characteristic color only on freshly broken surfaces or only on weathered surfaces. Some—precious opals, for example—show a stunning display of colors on reflecting surfaces. Others change color slightly with a change in the angle of the light shining on their surfaces. Many ionically bonded crystals are colorless.
Streak refers to the color of the fine deposit of mineral powder left on an abrasive surface, such as a tile of unglazed porcelain, when a mineral is scraped across it. Such a tile, called a streak plate (Figure 3.20), is a good identification tool because the uniformly small grains of the mineral that are present in the powder are revealed on the plate. Hematite, for example, may look black, red, or brown, but this mineral will always leave a trail of reddish brown powder on a streak plate.

Color is a complex and not yet fully understood property of minerals. It is determined both by the kinds of ions found in the pure mineral and by trace elements.
Ions and Mineral Color
The color of pure minerals depends on the presence of certain ions, such as iron or chromium, that strongly absorb portions of the light spectrum. Olivine that contains iron, for example, absorbs all colors except green, which it reflects, so we see this type of olivine as green. We see pure magnesium olivine as white (transparent and colorless).
Trace Elements and Mineral Color
All minerals contain impurities. Instruments can now measure even very small quantities of some elements—as little as a billionth of a gram in some cases. Elements that make up much less than 0.1 percent of a mineral are referred to as trace elements.
Some trace elements can be used to deduce the origins of the minerals in which they are found. Others, such as the traces of uranium in some granites, contribute to local natural radioactivity. Still others, such as small dispersed flakes of hematite that color a feldspar crystal brownish or reddish, are notable because they give a general color to an otherwise colorless mineral. Many of the gem varieties of minerals, such as emerald (green beryl) and sapphire (blue corundum), get their color from trace elements dissolved in the solid crystal (Figure 3.21). Emerald derives its color from chromium; the sources of sapphire’s blue color are iron and titanium.

73
Density
You can easily feel the difference in weight between a piece of hematite and a piece of sulfur of the same size by lifting the two pieces. A great many common rock-forming minerals, however, are too similar in density for such a simple test. Scientists therefore need some easy method to measure this property of minerals. A standard measure of density is specific gravity, which is the weight of a mineral divided by the weight of an equal volume of pure water at 4°C.
Density depends on the atomic mass of a mineral’s atoms or ions and how closely they are packed in its crystal structure. Consider magnetite, an iron oxide with a density of 5.2 g/cm3. Its high density results partly from the high atomic mass of iron and partly from the closely packed structure that magnetite shares with the other members of the spinel group of oxides. The density of iron olivine, at 4.4 g/cm3, is lower than that of magnetite for two reasons. First, the atomic mass of silicon, one of the elements that make up olivines, is lower than that of iron. Second, iron olivine has a more openly packed structure than minerals of the spinel group. The density of magnesium olivine is even lower, at 3.32 g/cm3, because magnesium’s atomic mass is much lower than that of iron.
Increases in density caused by increases in pressure affect the way minerals transmit light, heat, and seismic waves. Experiments at extremely high pressures have shown that the structure of olivine converts into the denser structure of spinel olivine at pressures corresponding to a depth in Earth’s mantle of 410 km. At a greater depth, 660 km, mantle materials are further transformed into silicate minerals with the even more densely packed structure of perovskite olivine. Because of the huge volume of the lower mantle, perovskite olivine is probably the most abundant mineral in Earth as a whole. Temperature also affects density: the higher the temperature, the more open and expanded the structure of the mineral, and thus the lower its density.
Crystal Habit
A mineral’s crystal habit is the shape in which individual crystals or aggregates of crystals grow. Some minerals have such a distinctive crystal habit that they are easily recognizable. An example is quartz, with its six-sided column topped by a pyramid-like set of faces (see Figure 3.7). Crystal habits are often named after common geometric shapes, such as blades, plates, and needles. These shapes indicate not only the planes of the mineral’s crystal structure, but also the typical speed and direction of crystal growth. Thus, a needlelike crystal is one that grows very quickly in one direction and very slowly in all other directions. In contrast, a plate-shaped crystal (often referred to as platy) grows fast in all directions that are perpendicular to its single direction of slow growth. Fibrous crystals take shape as multiple long, narrow fibers, essentially aggregates of long needles. Asbestos is a generic name for a group of silicate minerals with a more or less fibrous habit that allows the crystals to become embedded in the lungs if they are inhaled (Figure 3.22).

Table 3.4 summarizes the physical properties of minerals that we have discussed in this section.
74
| Property | Relation to Composition and Crystal Structure |
|---|---|
| Hardness | Strong chemical bonds result in hard minerals. Covalently bonded minerals are generally harder than ionically bonded minerals. |
| Cleavage | Cleavage is poor if bonds in crystal structure are strong, good if bonds are weak. Covalent bonds generally give poor or no cleavage; ionic bonds are weaker and so give good cleavage. |
| Fracture | Related to distribution of bond strengths across irregular surfaces other than cleavage planes. |
| Luster | Tends to be glassy for ionically bonded crystals, more variable for covalently bonded crystals. |
| Color | Determined by ions and trace elements. Many ionically bonded crystals are colorless. Iron tends to color strongly. |
| Streak | Color of fine mineral powder is more characteristic than that of massive mineral because of uniformly small size of grains. |
| Density | Depends on atomic weight of atoms or ions and their closeness of packing in crystal structure. |
| Crystal habit | Depends on the planes of a mineral’s crystal structure and the typical speed and direction of crystal growth. |