Concentrations of Valuable Mineral Resources
The rock cycle turns out to be crucial in creating economically important concentrations of the many valuable minerals found in Earth’s crust. Minerals are not only sources of metals, which will be our focus here, but also provide us with stone for buildings and roads, phosphates for fertilizers, cement for construction, clays for ceramics, sand for silicon chips and fiber-optic cables, and many other items we use in our daily lives. Finding these minerals and extracting them is a vital job for Earth scientists, so we turn our attention next to how and where some of these geologic prizes are formed.
The chemical elements of Earth’s crust are widely distributed in many kinds of minerals, and those minerals are found in a great variety of rocks. In most places, any given element will be found homogenized with other elements in amounts close to its average concentration in the crust. An ordinary granitic rock, for example, may contain a small percentage of iron, close to the average concentration of iron in Earth’s crust.
When an element is present in concentrations higher than the average, it means that the rock underwent some geologic process that concentrated larger quantities of that element than normal. The concentration factor of an element in a mineral deposit is the ratio of the element’s abundance in the deposit to its average abundance in the crust. High concentrations of elements are found in a limited number of specific geologic settings. These settings are of economic interest because the higher the concentration of a resource in a given deposit, the lower the cost to recover it.
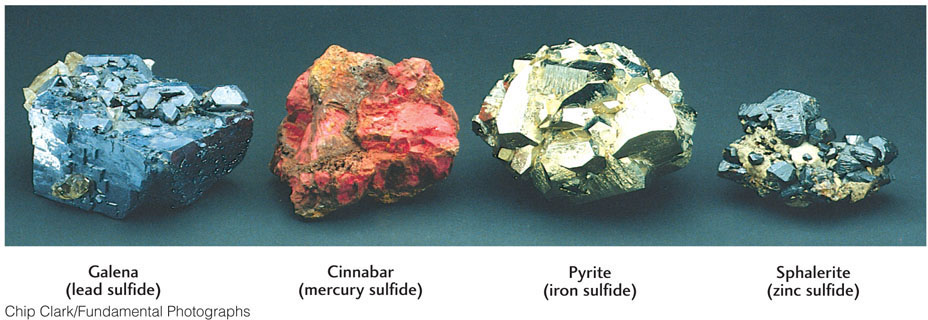
Ores are rich deposits of minerals from which valuable metals can be recovered profitably (see Practicing Geology). The minerals containing these metals are referred to as ore minerals. Ore minerals include sulfides (the largest group), oxides, and silicates. The ore minerals in each of these groups are compounds of metallic elements with sulfur, with oxygen, and with silicon and oxygen, respectively. The copper ore mineral covelite, for example, is a copper sulfide (CuS). The iron ore mineral hematite (Fe2O3) is an iron oxide. The nickel ore mineral garnierite is a nickel silicate (Ni3Si2O5(OH)4). In addition, some metals, such as gold, are found in their native state—that is, uncombined with other elements (Figure 3.29).

82
Hydrothermal Deposits
Many of the most valuable ores are formed in regions of volcanism by the interaction of igneous processes with the hydrosphere. Recall from our discussion of the rock cycle that subduction zones may be associated with the melting of oceanic lithosphere to form igneous rocks. Very large ore deposits can be formed in such plate tectonic settings when hot water solutions—also known as hydrothermal solutions—are formed around bodies of molten rock. This happens when circulating groundwater or seawater comes into contact with a magmatic intrusion, reacts with it, and carries off significant quantities of elements and ions released by the reaction. These elements and ions then interact with one another to form ore minerals, usually as the solution cools.
Veins
Hydrothermal solutions moving through rocks often deposit ore minerals (Figure 3.30). These fluids flow easily through fractures in the rocks, cooling rapidly in the process. Quick cooling causes rapid precipitation of the ore minerals. The resulting tabular (sheetlike) deposits of precipitated minerals in the fractures are called veins. Some ore minerals are found in veins; others are found in the rocks surrounding the veins, which are altered when the hydrothermal solutions heat and infiltrate those rocks. As the solutions react with the surrounding rocks, they may precipitate ore minerals together with quartz, calcite, or other common vein-filling minerals. Vein deposits are a major source of gold.
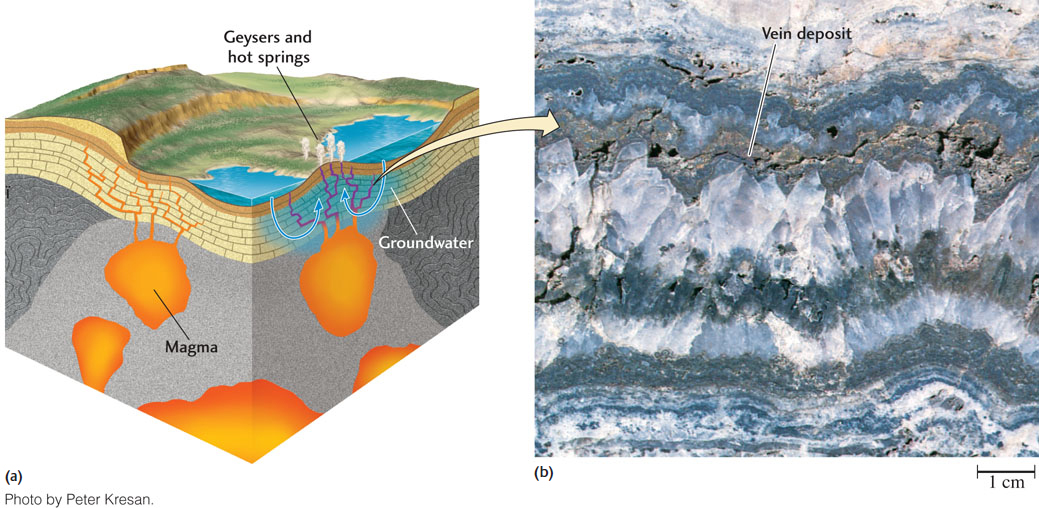
Hydrothermal vein deposits are among the most important sources of metallic ores. Typically, metallic ores exist as sulfides, such as iron sulfide (pyrite), lead sulfide (galena), zinc sulfide (sphalerite), and mercury sulfide (cinnabar) (Figure 3.31). Hydrothermal solutions reach the surface as hot springs and geysers, many of which precipitate metallic ores—including ores of lead, zinc, and mercury—as they cool.
Disseminated Deposits
Deposits of ore minerals that are scattered through volumes of rock much larger than veins are called disseminated deposits. In both igneous and sedimentary rocks, minerals are disseminated along abundant cracks and fractures. Among the economically important disseminated deposits are the copper deposits of Chile and the southwestern United States. These deposits develop in geologic regions with abundant igneous rocks, usually emplaced as large intrusive bodies. In Chile, these intrusive igneous rocks are related to the subduction of oceanic lithosphere beneath the Andes (an event very similar to what was described in our example of the rock cycle). The most common copper mineral in these deposits is chalcopyrite, a copper sulfide (Figure 3.32). The copper was deposited when ore minerals were introduced into a great number of tiny fractures in granitic intrusive rocks and in the rocks surrounding the upper parts of the igneous intrusions. Some unknown process associated with the magmatic intrusion or its aftermath broke these rocks into millions of pieces. Hydrothermal solutions penetrated and re-cemented the rocks by precipitating ore minerals throughout the extensive network of tiny fractures. This widespread dispersal produced a low-grade but very large resource of many millions of tons of ore, which can be mined economically by large-scale methods (Figure 3.33).


83
The lead-zinc deposits of the Upper Mississippi Valley, which extend from southwestern Wisconsin to Kansas and Oklahoma, are found in sedimentary rocks. The ores in this disseminated hydrothermal deposit are not associated with a known magmatic intrusion that could have been a source of hydrothermal solutions, so their origin must be very different. Some geologists speculate that the ores were deposited by groundwater that was driven out of the ancestral Appalachian Mountains when they were much higher. A continent-continent collision between North America and Africa may have created a continental-scale squeegee that pushed fluids from deep within the collision zone all the way into the continental interior of North America. Groundwater may have penetrated hot crustal rocks at great depths and dissolved soluble ore minerals, then moved upward into the overlying sedimentary rocks, where it precipitated the minerals as fillings in cavities. In some cases, it appears that these solutions infiltrated limestone formations and dissolved some carbonates, then replaced the carbonates with equal volumes of sulfide crystals. The major minerals of these deposits are lead sulfide (galena) and zinc sulfide (sphalerite).
Igneous Deposits
The most important deposits of ore minerals in igneous rocks are found as segregations of ore minerals near the bottoms of magmatic intrusions (see Chapter 5, Practicing Geology). These deposits form when minerals with relatively high melting temperatures crystallize from a body of cooling magma, settle, and accumulate at the base of the magma. Most of the chromium and platinum ores of the world, such as the deposits in South Africa and Montana, are found as layered accumulations of minerals that formed in this way (Figure 3.34). One of the richest ore deposits ever found, at Sudbury, Ontario, is a large igneous intrusion containing great quantities of layered nickel, copper, and iron sulfides near its base. Geologists believe that these sulfide deposits formed from the crystallization of a dense, sulfide-rich liquid that separated from the rest of a cooling magmatic intrusion and sank to the bottom before it congealed.

84
As the magma in a large granite-forming intrusion cools, the last material to crystallize forms pegmatites, extremely coarse-grained rocks in which minerals present in only trace amounts in the magma are concentrated. Pegmatites may contain rare ore minerals rich in elements such as beryllium, boron, fluorine, lithium, niobium, and uranium, as well as gem minerals such as tourmaline.
Sedimentary Deposits
Sedimentary deposits include some of the world’s most valuable mineral sources. Many economically important minerals, such as copper, iron, and other metals, segregate as an ordinary result of sedimentary processes. These deposits are chemically precipitated in sedimentary environments to which large quantities of metals are transported in solution. Some of the important sedimentary copper ores, such as those of the Permian Kupferschiefer (German for “copper slate”) beds of Germany, may have precipitated from hydrothermal solutions rich in metal sulfides that interacted with sediments on the seafloor. The plate tectonic setting of these deposits may have been something like the mid-ocean ridge described in our example of the rock cycle, except that it developed within a continent. Here, rifting of the continental crust led to development of a deep trough, where sediments and ore minerals were deposited in a very still, narrow sea.
85
Many rich deposits of gold, diamonds, and other heavy minerals such as magnetite and chromite are found in placers, sedimentary ore deposits that have been concentrated by the mechanical sorting action of river currents. These ore deposits originate where uplifted rocks weather to form grains of sediment, which are then sorted by weight when currents of water flow over them. Because heavy minerals settle out of a current more quickly than lighter minerals such as quartz and feldspar, they tend to accumulate on streambeds and sandbars. Similarly, ocean waves preferentially deposit heavy minerals on beaches or shallow offshore bars. A gold panner accomplishes the same thing: the shaking of a water-filled pan allows the lighter minerals to be washed away, leaving the heavier gold in the bottom of the pan (Figure 3.35).

Some placers can be traced upstream to the location of the original mineral deposit, usually of igneous origin, from which the minerals were eroded. Erosion of the Mother Lode, an extensive gold-bearing vein system lying along the western flanks of the Sierra Nevada, produced the placers that were discovered in 1848 and led to the California gold rush. The placers were found before their source was discovered. Placers also led to the discovery of the Kimberley diamond mines of South Africa two decades later.