Classification of Chemical and Biological Sediments and Sedimentary Rocks
Chemical and biological sediments and sedimentary rocks can be classified by their chemical composition (Table 5.4). Geologists distinguish between chemical sediments and biological sediments not only for convenience, but also to emphasize the importance of organisms as the chief mediators of biological sedimentation. Both kinds of sediments can tell us about chemical conditions in the ocean, their predominant environment of deposition.
| Sediment | Rock | Chemical Composition | Minerals |
|---|---|---|---|
| Biological | |||
| Sand and mud (primarily bioclastic) | Limestone | Calcium carbonate (CaCO3) | Calcite, aragonite |
| Siliceous sediment | Chert | Silica (SiO2) | Opal, chaldeony, quartz |
| Peat, organic matter | Organics | Carbon compounds; carbon compounded with oxygen and hydrogen | (Coal, oil, natural gas) |
| No primary sediment (formed by diagenesis) | Phosphorite | Calcium phosphate (Ca3(PO4)2) | Apatite |
| Chemical | |||
| No primary sediment (formed by diagenesis) | Dolostone | Calcium-magnesium carbonate (CaMg(CO3)2) | Dolomite |
| Iron oxide sediment | Iron formation | Iron silicate; oxide (Fe2O3);limonite, carbonate | Hematite, siderite |
| Evaporite sediment | Evaporite | Calcium sulfate (CaSO4); sodium chloride (NaCl) | Gypsum, anhydrite, halite, other salts |
136
Carbonate Sediments and Rocks
Most carbonate sediments and carbonate rocks are formed by the accumulation and lithification of carbonate minerals that are directly or indirectly precipitated by organisms. The most abundant of these carbonate minerals is calcite (calcium carbonate, CaCO3); in addition, most carbonate sediments contain aragonite, a less stable form of calcium carbonate. Some organisms precipitate calcite, some precipitate aragonite, and some precipitate both. During burial and diagenesis, carbonate sediments react with water to form a new suite of carbonate minerals.
The dominant biological sedimentary rock lithified from carbonate sediments is limestone, which is composed mainly of calcite (Figure 5.22a). Limestone is formed from carbonate sands and muds and, in some cases, ancient reefs (see Figure 5.10).

Another abundant carbonate rock is dolostone, made up of the mineral dolomite, which is composed of calcium–magnesium carbonate. Dolostones are diagenetically altered carbonate sediments and limestones. Dolomite does not form as a primary precipitate from ordinary seawater, and no organisms secrete shells of dolomite. Instead, some calcium ions in the calcite or aragonite of a carbonate sediment are exchanged for magnesium ions from seawater (or magnesium-rich groundwater) slowly passing through the pores of the sediment. This exchange converts calcium carbonate (CaCO3) into dolomite (CaMg(CO3)2).
Direct Biological Precipitation of Carbonate Sediments
Carbonate rocks are abundant because of the large amounts of calcium and carbonate minerals dissolved in seawater, which organisms can convert directly into shells. Calcium is supplied by the weathering of feldspars and other minerals in igneous and metamorphic rocks. Carbonate minerals are derived from the carbon dioxide in the atmosphere. Calcium and carbonate minerals also come from the easily weathered limestone on the continents.
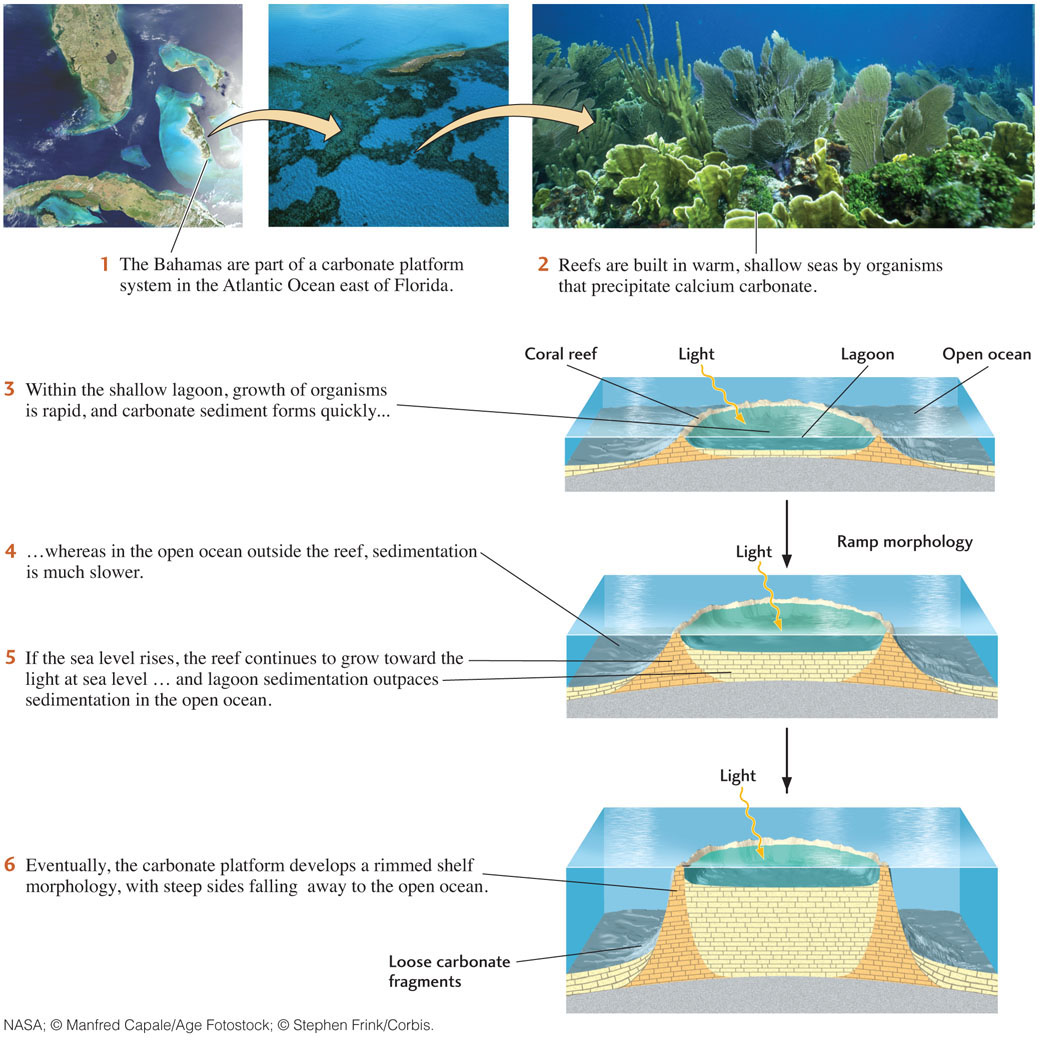
Most carbonate sediments of shallow marine environments are bioclastic sediments originally secreted as shells by organisms living near the surface or on the bottom. After the organisms die, they break apart, producing shells or fragments of shells that constitute individual particles, or clasts, of carbonate sediment. These sediments are found in tropical and subtropical environments from Pacific islands to the Caribbean and the Bahamas. Carbonate sediments are most accessible for study in these spectacular vacation spots, but the deep sea is where most carbonate sediments are deposited today.
Most of the carbonate sediments deposited on the abyssal plain of the deep sea are derived from the calcite shells of foraminifera (see Figure 3.1b) and other planktonic organisms that live in the surface waters and secrete calcium carbonate. When the organisms die, their shells settle to the seafloor and accumulate there as sediments.
137
Reefs are moundlike or ridgelike organic structures composed of the carbonate skeletons and shells of millions of organisms. In the warm seas of the present, reefs are built mainly by corals, but hundreds of other organisms, such as algae, clams, and snails, also contribute. In contrast to the soft, loose sediments produced in other carbonate environments, the reef forms a rigid, wave-resistant structure of solid calcite and aragonite that is built up to and slightly above sea level. The solid calcite and aragonite of the reef is produced directly by the carbonate-cementing action of the organisms; there is no loose sediment stage.
Coral reefs may give rise to carbonate platforms: extensive flat, shallow areas, such as the Bahamas, where both biological and nonbiological carbonate sediments are deposited (Figure 5.23). Carbonate platforms are among the most important carbonate environments, both in past geologic ages and at present. The building of a carbonate platform results from interactions between the biosphere, hydrosphere, and lithosphere (see Earth Issues 5.1). The process begins with a reef that encloses and shelters an area of shallow ocean water known as a lagoon. Carbonate-secreting organisms proliferate in and around the lagoon, and carbonate sediments accumulate rapidly, while in the open ocean outside the reef, sedimentation is much slower. At this point, the carbonate platform has a ramp morphology, with gentle slopes leading to deeper water. As sedimentation in the lagoon continues to outpace that outside the reef, the platform grows taller, developing a rimmed shelf morphology. Below the rims are steep slopes covered with loose carbonate sediments derived from the rim materials.
Reefs and Evolutionary Processes
Today, reefs are constructed mainly by corals, but at earlier times in Earth’s history, they were constructed by other organisms, such as a now-extinct variety of mollusk (Figure 5.24). Carbonate sediments and rocks formed from reefs record the diversification and extinction of reef-building organisms over geologic time. That record shows us how ecology and environmental change help to regulate the process of evolution.

138
Today, natural and anthropogenic changes threaten the growth of coral reefs, which are very sensitive to environmental change. In 1998, an El Niño event (described in Chapter 15) raised sea surface temperatures so much that many reefs in the western Indian Ocean were killed. The reefs of the Florida Keys are dying off for a completely different reason: they are getting too much of a good thing. Groundwaters originating in the farmlands of the Florida Peninsula are seeping out to the reefs and exposing them to lethal concentrations of nutrients.
Indirect Biological Precipitation of Carbonate Sediments
A significant fraction of the carbonate mud deposited in lagoons and on shallow carbonate platforms is precipitated indirectly from seawater. Microorganisms may be involved in this process, but their role is still uncertain. They may help to shift the balance of calcium (Ca2+) and carbonate (CO32−) ions in the seawater surrounding them so that calcium carbonate (CaCO3) is formed. Microorganisms can precipitate carbonate minerals only if their external environment already contains abundant calcium and carbonate ions. In this case, chemicals that the microorganisms emit into the seawater cause the minerals to precipitate. In contrast, shelled organisms secrete carbonate minerals continually as a normal part of their life cycle.
139
Earth Issues: 5.1 Darwin’s Coral Reefs and Atolls
For more than 200 years, coral reefs have attracted explorers and travel writers. Ever since Charles Darwin sailed the oceans on the Beagle from 1831 to 1836, these reefs have been a matter of scientific discussion as well. Darwin was one of the first scientists to analyze the geology of coral reefs, and his theory of the origin of one type of coral reef is still accepted today.
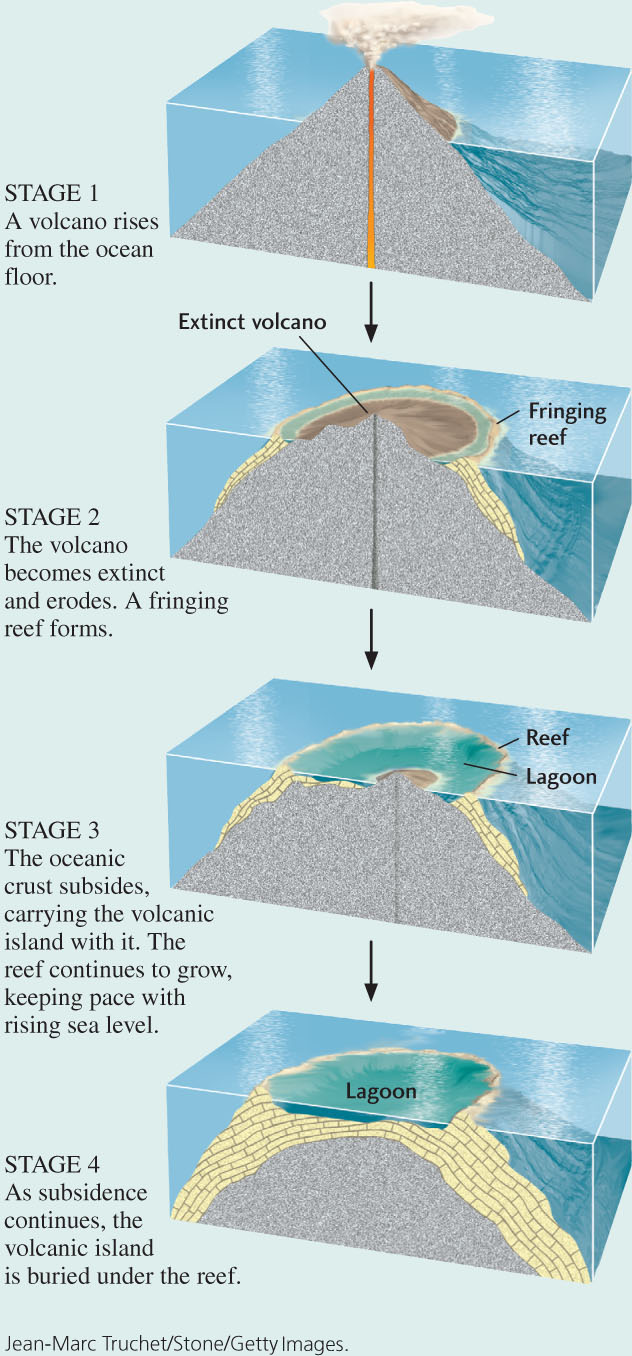
The coral reefs that Darwin studied were atolls, coral islands in the open ocean surrounding circular lagoons. The outermost part of an atoll is a slightly submerged, wave-resistant reef front: a steep slope facing the ocean. The reef front is composed of the interlaced skeletons of corals and calcareous algae, which form a tough, hard limestone. Behind the reef front is a flat platform extending into a shallow lagoon. An island may lie at the center of the lagoon. Parts of the reef, as well as the central island, are above sea level and may become forested. A great many plant and animal species inhabit the reef and the lagoon.
Coral reefs are generally limited to waters less than 20 m deep because, below that depth, seawater does not transmit enough light to enable reef-building organisms to grow. How, then, could an atoll be built up from the bottom of the deep, dark ocean? Darwin proposed that the process starts with a volcano building up to the sea surface from the seafloor and forming an island. As the volcano becomes dormant, temporarily or permanently, corals and algae colonize the shore of the island and build fringing reefs. Erosion may then lower the volcanic island almost to sea level.

Darwin reasoned that if such a volcanic island were to subside slowly beneath the waves, actively growing corals and algae might keep pace with its subsidence, continuously building up the reef over geologic time. In this way, the volcanic island would disappear, leaving an atoll in its place. More than 100 years after Darwin proposed his theory, deep drilling on several atolls found volcanic rock below their coralline limestone. And, some decades later, the theory of plate tectonics explained both volcanism and the subsidence that resulted from plate cooling and contraction.
140
Evaporite Sediments and Rocks: Products of Evaporation
Evaporite sediments and evaporite rocks are chemically precipitated from evaporating seawater or, in some cases, lake water.
Marine Evaporites
Marine evaporites are chemical sediments and sedimentary rocks formed by the evaporation of seawater. These sediments and rocks contain minerals formed by the crystallization of sodium chloride (halite), calcium sulfate (gypsum and anhydrite), and other combinations of ions commonly found in seawater. As evaporation proceeds and the ions in the seawater become more concentrated, those minerals crystallize in a set sequence. As dissolved ions precipitate to form each mineral, the composition of the evaporating seawater changes.
Seawater has the same composition in all the oceans, which explains why marine evaporites are so similar the world over. No matter where seawater evaporates, the same sequence of minerals always forms. The study of evaporite sediments also shows us that the composition of the oceans has stayed more or less constant over the past 1.8 billion years. Before that time, however, the precipitation sequence may have been different, indicating that the composition of seawater may also have been different.
The great volume of many marine evaporites, some of which are hundreds of meters thick, shows that they could not have formed from the small amount of water that could be held in a small, shallow bay or pond. A huge amount of seawater must have evaporated to form them. The way in which such large quantities of seawater evaporate is very clear in bays or arms of the sea that meet the following conditions (Figure 5.25):
 The freshwater supply from rivers is small.
The freshwater supply from rivers is small. Connections to the open ocean are constricted.
Connections to the open ocean are constricted. The climate is arid.
The climate is arid.
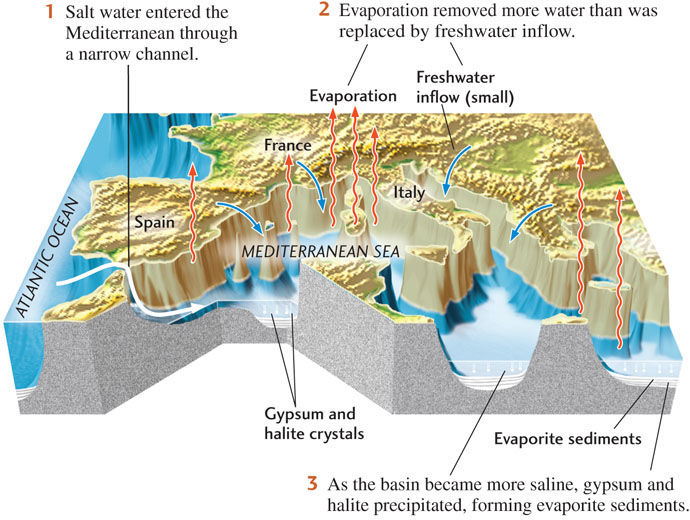
In such locations, water evaporates steadily, but the connections allow seawater to flow in to replenish the evaporating waters of the bay. As a result, those waters stay at a constant volume, but become more saline than the open ocean. The evaporating bay waters remain more or less constantly supersaturated and steadily deposit evaporite minerals on the floor of the bay.
As seawater evaporates, the first precipitates to form are the carbonates. Continued evaporation leads to the precipitation of gypsum, or calcium sulfate (CaSO4 • 2H2O) (Figure 5.22b). By the time gypsum precipitates, almost no carbonate ions are left in the water. Gypsum is the principal component of plaster of Paris and is used in the manufacture of wallboard, which lines the walls of most new houses.
After still further evaporation, the mineral halite, or sodium chloride (NaCl)—one of the most common chemical sediments precipitated from evaporating seawater—starts to form (Figure 5.22c). Halite, as you may remember from Chapter 3, is table salt. Deep under the city of Detroit, Michigan, beds of salt laid down by an evaporating arm of an ancient ocean are commercially mined.
141
In the final stages of evaporation, after the sodium chloride is gone, magnesium and potassium chlorides and sulfates precipitate from the water. The salt mines near Carlsbad, New Mexico, contain commercial quantities of potassium chloride. Potassium chloride is often used as a substitute for table salt by people with certain dietary restrictions.
This sequence of mineral precipitation from seawater has been studied in the laboratory and is matched by the bedding sequences found in certain natural evaporite formations. Most of the world’s evaporites consist of thick sequences of dolomite, gypsum, and halite and do not contain the final-stage precipitates. Many do not go even as far as halite. The absence of the final stages indicates that the water did not evaporate completely, but was replenished by normal seawater as evaporation continued.
Nonmarine Evaporites
Evaporite sediments also form in arid-region lakes that typically have few or no river outlets. In such lakes, evaporation controls the lake level, and incoming minerals derived from chemical weathering accumulate as sediments. The Great Salt Lake is one of the best known of these lakes (Figure 5.26). In the dry climate of Utah, evaporation has more than balanced the inflow of fresh water from rivers and rain. As a result, the concentrations of dissolved ions in the lake make it one of the saltiest bodies of water in the world—eight times more saline than seawater. Sediments form when these ions precipitate.

142
Small lakes in arid regions may precipitate unusual salts, such as borates (compounds of the element boron), and some become alkaline. The water in this kind of lake is poisonous. Economically valuable deposits of borates and nitrates (minerals containing the element nitrogen) are found in the sediments beneath some of these lakes.
Other Biological and Chemical Sediments
Carbonate minerals secreted by organisms are the principal source of biological sediments, and minerals precipitated from evaporating seawater are the principal source of chemical sediments. However, there are several less common biological and chemical sediments that are locally abundant. They include chert, coal, phosphorite, iron ore, and the organic carbon–rich sediments that produce oil and natural gas. The role of biological versus chemical processes in forming these sediments is variable.
143
Siliceous Sediments: Sources of Chert
One of the first sedimentary rocks to be used for practical purposes by our prehistoric ancestors was chert, which is composed of silica (SiO2) (Figure 5.22d). Early hunters used it for arrowheads and other tools because it could be chipped and shaped to form hard, sharp implements. A common name for chert is flint; the two terms are virtually interchangeable. The silica in most cherts is in the form of extremely fine grained quartz. Some geologically young cherts consist of opal, a less fine grained form of silica.
Like calcium carbonate sediments, many siliceous sediments are precipitated biologically as silica shells secreted by planktonic organisms that settle to the deep seafloor and accumulate as layers of sediment. After these sediments are buried by later sediments, they are cemented into chert. Chert may also form as nodules and irregular masses replacing carbonate in limestones and dolostones.
144
Phosphorite Sediments
Among the many other kinds of chemical and biological sediments deposited in the ocean is phosphorite. Sometimes called phosphate rock, phosphorite is composed of calcium phosphate precipitated from phosphate-rich seawater in places where currents of deep, cold water containing phosphate and other nutrients rise along continental margins. Organisms play an important role in creating phosphate-rich water, and bacteria that live on sulfur may play a key role in precipitating phosphate minerals. The phosphorite forms diagenetically by the interaction of calcium phosphate with muddy or carbonate sediments.
Iron Oxide Sediments: Source of Iron Formations
Iron formations are sedimentary rocks that usually contain more than 15 percent iron in the form of iron oxides and some iron silicates and iron carbonates. Iron oxides were once thought to be of chemical origin, but there is now some evidence that they may have been precipitated indirectly by microorganisms. Most of these rocks formed early in Earth’s history, when there was less oxygen in the atmosphere and, as a result, iron dissolved more easily. Iron was transported to the ocean in soluble form, and where microorganisms were producing oxygen, it reacted with that oxygen and precipitated from solution as iron oxides (see Chapter 11).
Organic Sediments: Sources of Coal, Oil, and Natural Gas
Coal is a biological sedimentary rock composed almost entirely of organic carbon and formed by the diagenesis of wetland vegetation. In wetland environments, vegetation may be preserved from decay and accumulate as a rich organic material called peat, which contains more than 50 percent carbon. If peat is ultimately buried, it may be transformed into coal. Coal is classified as an organic sedimentary rock, a class that consists entirely or partly of organic carbon–rich deposits formed by the diagenesis of once-living material that has been buried.
In both lake and ocean waters, the remains of algae, bacteria, and other microscopic organisms may accumulate in fine-grained sediments as organic matter that can be transformed by diagenesis into oil and natural gas. Crude oil (petroleum) and natural gas are fluids that are not normally classed with sedimentary rocks. They can be considered organic sediments, however, because they form by the diagenesis of organic material in the pores of sedimentary rocks. Deep burial changes the organic matter originally deposited along with inorganic sediments into a fluid that then escapes to porous rock formations and becomes trapped there. Oil and natural gas are found mainly in sandstones and limestones.
As supplies of oil and natural gas begin to diminish, the challenges for geologists increase. These challenges include finding new oil fields as well as squeezing out what is left behind in existing fields. Ultimately, it is the availability of organic sediments that limits how much oil and gas can be found. These sediments were more abundant in some periods of Earth’s history, and they were formed more easily in certain parts of the world. So there are geologic constraints that we must learn to accept. But we can learn to be smarter about how we explore for what little oil is left, and the need for well-trained geologists has never been greater.