TOOLS OF THE ASTRONOMER’S TRADE
 Kinetic Energy, Temperature, and Whether Planets Have Atmospheres
Kinetic Energy, Temperature, and Whether Planets Have Atmospheres
Amoving object possesses energy as a result of its motion. The faster it moves, the more energy it has (see Figure 4-20). Energy of this type is called kinetic energy. If an object of mass m is moving with a speed ν its kinetic energy Ek is given by

This expression for kinetic energy is valid for all objects, both big and small, from atoms and molecules to planets and stars, as long as their speeds are slow in relation to the speed of light. If the mass is expressed in kilograms and the speed in meters per second, the kinetic energy is expressed in joules (J).
EXAMPLE: An automobile of mass 1000 kg driving at a typical freeway speed of 30 m/s (= 108 km/h = 67 mi/h) has a kinetic energy of
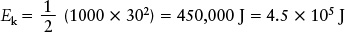
Consider a gas, such as the atmosphere of a star or planet. Some of the gas atoms or molecules will be moving slowly, with little kinetic energy, while others will be moving faster and have more kinetic energy. The temperature of the gas is a direct measure of the average amount of kinetic energy per atom or molecule. The hotter the gas, the faster atoms or molecules move, on average, and the greater the average kinetic energy of an atom or molecule (see Figure 5-11).
If the gas temperature is sufficiently high, typically several thousand kelvins, molecules move so fast that when they collide with one another, the energy of the collision can break the molecules apart into their constituent atoms. Thus, the Sun’s atmosphere, where the temperature is 5800 K, consists primarily of individual hydrogen atoms rather than hydrogen molecules. By contrast, the hydrogen atoms in Earth’s atmosphere (temperature 290 K) are combined with oxygen atoms into molecules of water vapor (H2O).
The physics of gases tells us that in a gas of temperature T (in kelvins), the average kinetic energy of an atom or molecule is
Kinetic energy of a gas atom or molecule
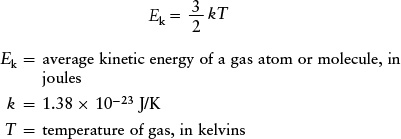
The quantity k is called the Boltzmann constant. Note that the higher the gas temperature, the greater the average kinetic energy of an atom or molecule of the gas. This average kinetic energy becomes zero at absolute zero, or T = 0, the temperature at which molecular motion is at a minimum.
At a given temperature, all kinds of atoms and molecules will have the same average kinetic energy. But the average speed of a given kind of atom or molecule depends on the particle’s mass. To see this dependence on mass, note that the average kinetic energy of a gas atom or molecule can be written in two equivalent ways:
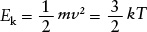
where ν represents the average speed of an atom or molecule in a gas with temperature T. Rearranging this equation, we obtain
Average speed of a gas atom or molecule
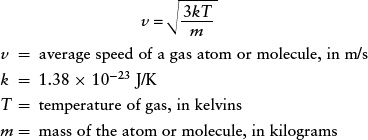
For a given gas temperature, the greater the mass of a given type of gas atom or molecule, the slower its average speed. (The value of v given by this equation is actually slightly higher than the average speed of the atoms or molecules in the gas, but it is close enough for our purposes here. If you are studying physics, you may know that v is actually the root-mean-square speed.)
183
EXAMPLE: What is the average speed of the oxygen molecules that you breathe at a room temperature of 20°C (= 68°F)?
Situation: We are given the temperature of a gas and are asked to find the average speed of the gas molecules.
Tools: We use the relationship 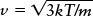 where T is the gas temperature in kelvins and m is the mass of a single oxygen molecule in kilograms.
where T is the gas temperature in kelvins and m is the mass of a single oxygen molecule in kilograms.
Answer: To use the equation to calculate the average speed ν, we must express the temperature T in kelvins (K) rather than degrees Celsius (°C). As we learned in Box 5-1, we do this by adding 273 to the Celsius temperature, so 20°C becomes (20 + 273) = 293 K. The mass m of an oxygen molecule is not given, but you can easily find that the mass of an oxygen atom is 2.66 × 10−26 kg. The mass of an oxygen molecule (O2) is twice the mass of an oxygen atom, or 2(2.66 × 10−26 kg) = 5.32 × 10−26 kg. Thus, the average speed of an oxygen molecule in 20°C air is
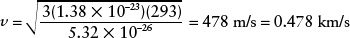
Review: This speed is about 1700 kilometers per hour, or about 1100 miles per hour. Hence, atoms and molecules move rapidly in even a moderate-temperature gas.
In some situations, atoms and molecules in a gas may be moving so fast that they can overcome the attractive force of a planet’s gravity and escape into interplanetary space. The minimum speed that an object at a planet’s surface must have in order to permanently leave the planet is called the planet’s escape speed (see Figure 4-22). The escape speed for a planet of mass M and radius R is given by
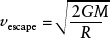
where G = 6.67 × 10−11 N m2/kg2 is the universal constant of gravitation.
The accompanying table gives the escape speed for the Sun, the planets, and the Moon. For example, to escape Earth, a cannon ball would have to be shot with an implausible speed greater than 11.2 km/s (25,100 mi/h).
A good rule of thumb is that a planet can retain a gas if the escape speed is at least 6 times greater than the average speed of the molecules in the gas. (Some molecules are moving slower than average, and others are moving faster, but very few are moving more than 6 times faster than average.) In such a case, very few molecules will be moving fast enough to escape from the planet’s gravity.
EXAMPLE: Consider Earth’s atmosphere. We saw that the average speed of oxygen molecules is 0.478 km/s at room temperature. The escape speed from Earth (11.2 km/s) is much more than 6 times the average speed of the oxygen molecules, so Earth has no trouble keeping oxygen in its atmosphere.
A similar calculation for hydrogen molecules (H2) gives a different result, however. At 293 K, the average speed of a hydrogen molecule is 1.9 km/s. Six times this speed is 11.4 km/s, which is about the escape speed from Earth. Thus, Earth does not retain hydrogen in its atmosphere. Any hydrogen released into the air slowly leaks away into space. On Jupiter, by contrast, the escape speed is so high that even the lightest gases such as hydrogen are retained in its atmosphere. But on Mercury the escape speed is low and the temperature high (so that gas molecules move faster), and Mercury cannot retain any significant atmosphere at all.
| Object | Escape speed (km/s) |
|---|---|
| Sun | 618.0 |
| Mercury | 4.3 |
| Venus | 10.4 |
| Earth | 11.2 |
| Moon | 2.4 |
| Mars | 5.0 |
| Jupiter | 59.5 |
| Saturn | 35.5 |
| Uranus | 21.3 |
| Neptune | 23.5 |