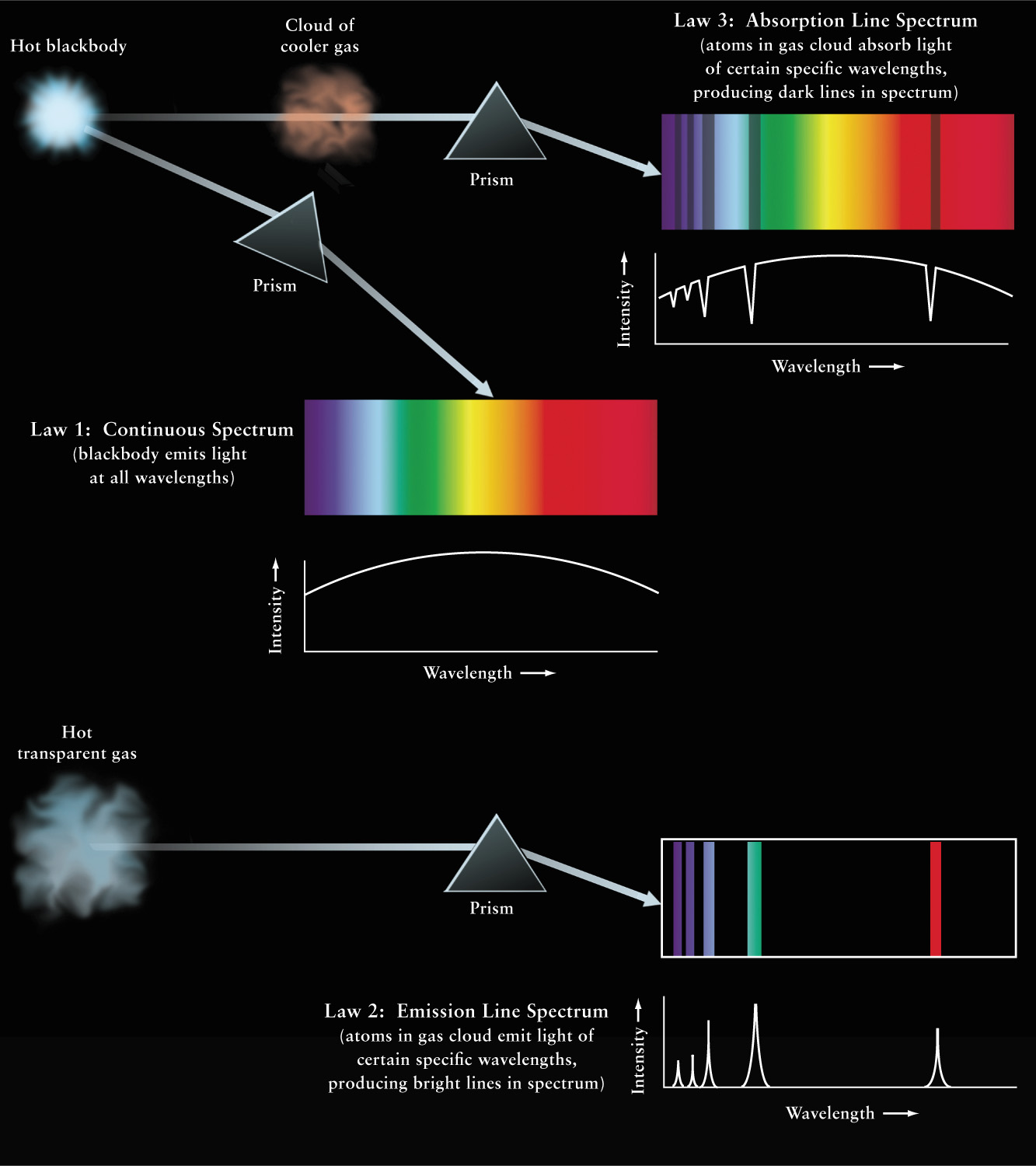
Figure 5-17: