5-2 Light is electromagnetic radiation and is characterized by its wavelength
Visible light, radio waves, and X-rays are all the same type of wave: They differ only in their wavelength
 Light is energy. This fact is apparent to anyone who has felt the warmth of the sunshine on a summer’s day. But what exactly is light? How is it produced? What is it made of? How does it move through space? Scholars have struggled with these questions throughout history.
Light is energy. This fact is apparent to anyone who has felt the warmth of the sunshine on a summer’s day. But what exactly is light? How is it produced? What is it made of? How does it move through space? Scholars have struggled with these questions throughout history.
Newton and the Nature of Color
The first major breakthrough in understanding light came from a simple experiment performed by Isaac Newton around 1670. Newton was familiar with what he called the “celebrated Phenomenon of Colours,” in which a beam of sunlight passing through a glass prism spreads out into the colors of the rainbow (Figure 5-3). This rainbow is called a spectrum (plural spectra).
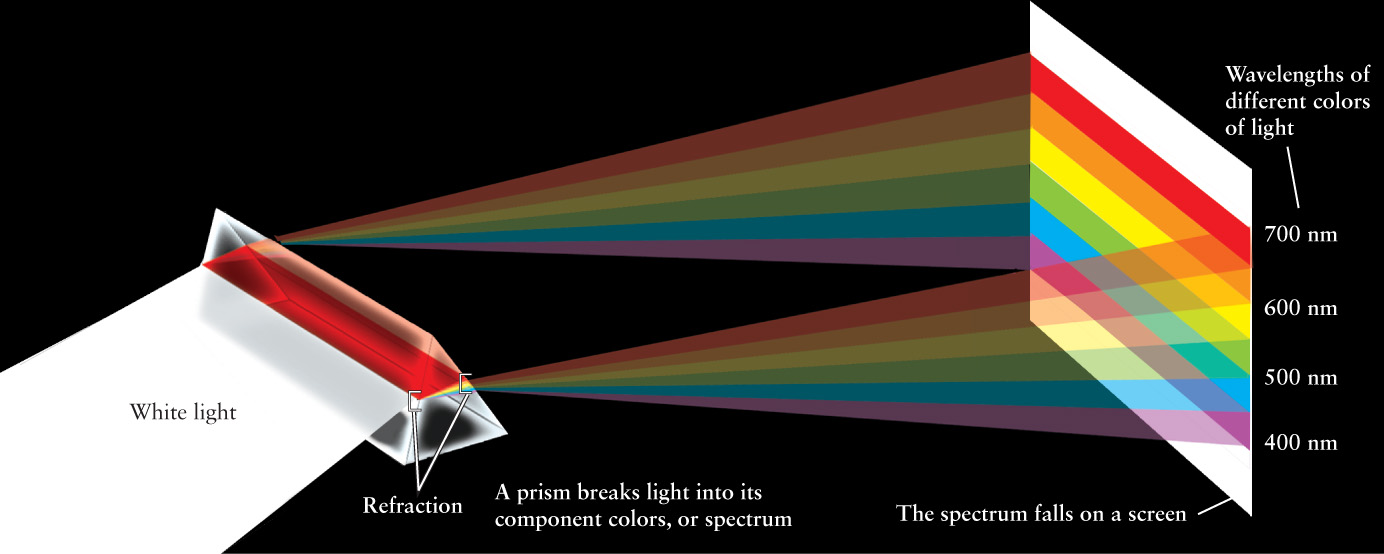
Until Newton’s time, it was thought that a prism somehow added colors to white light. To test this idea, Newton placed a second prism so that just one color of the spectrum passed through it: In Figure 5-4, only yellow light can pass through a slit to the second prism. According to the old theory, this should have caused a further change in the color of the light. But Newton found that each color of the spectrum was unchanged by the second prism; yellow remained yellow, blue remained blue, and so on. He concluded that a prism merely separates colors and does not add color. Hence, the spectrum produced by the first prism shows that sunlight is a mixture of all the colors of the rainbow. We often call sunlight “white light,” but keep in mind that white light is just our brain’s perception of sunlight’s mixed colors.
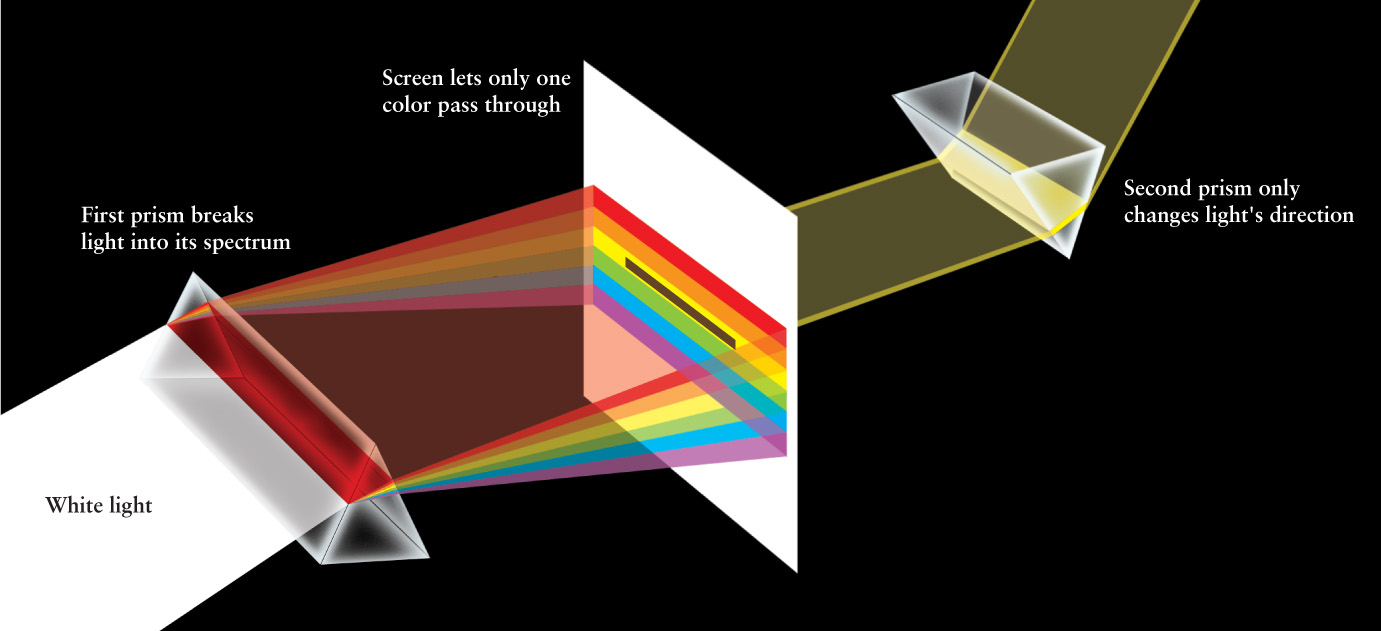
Newton suggested that light is made of particles too small to detect individually. In 1678, however, the Dutch physicist and astronomer Christiaan Huygens proposed a rival explanation. He suggested that light travels in the form of waves rather than particles.
CONCEPT CHECK 5-2
The rear brake lights on a car emit white light, but are covered with plastic that only allows red light to pass through. What color of plastic would the red light have to pass through in order to emerge as green light?
Young and the Wave Nature of Light
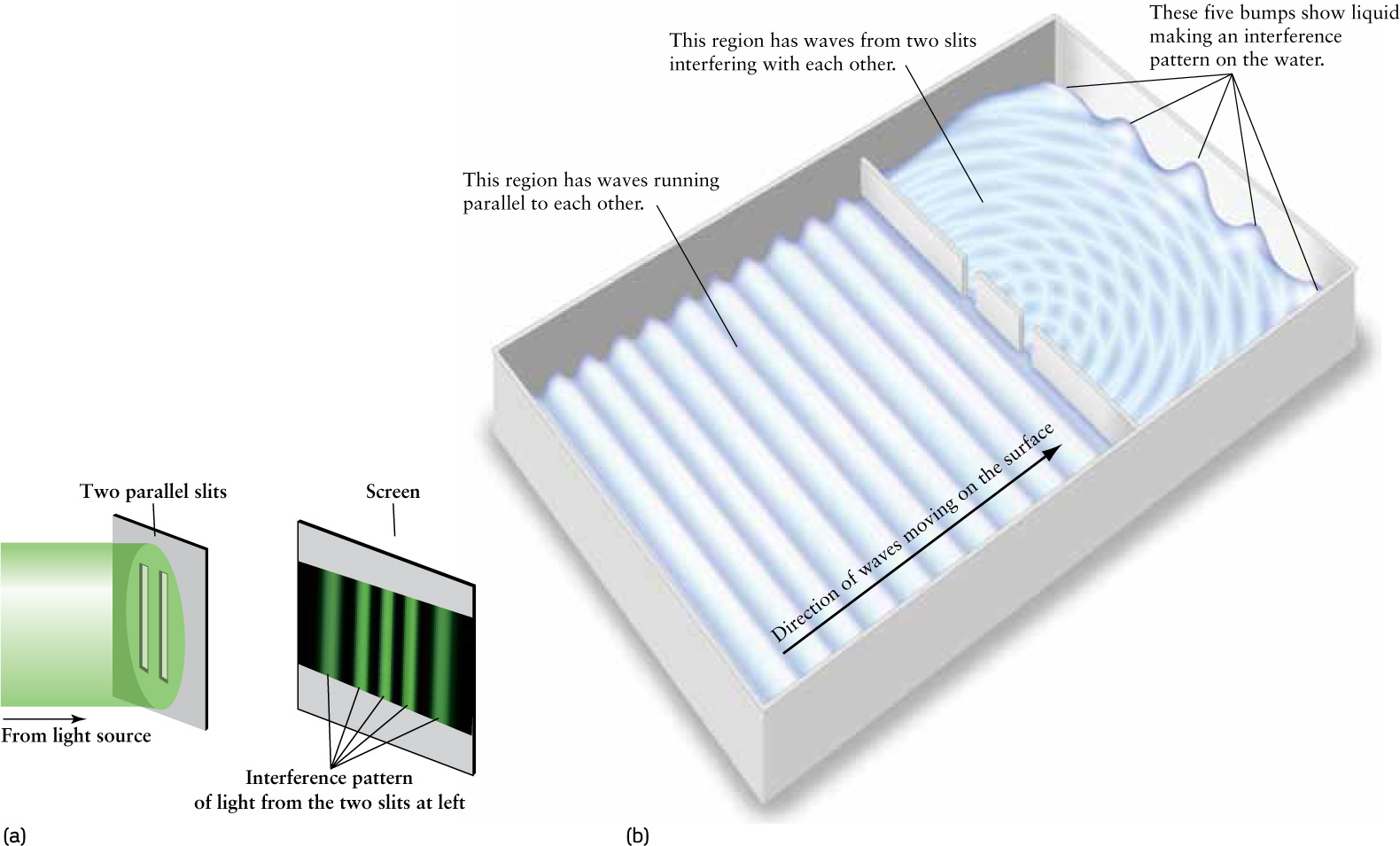
Around 1801, the English scientist Thomas Young carried out an experiment that convincingly demonstrated the wavelike aspect of light. He passed a beam of light through two thin, parallel slits in a plate, as shown in Figure 5-5a. On a white screen some distance beyond the slits, the light formed a pattern of alternating bright and dark bands. Young reasoned that if a beam of light was a stream of particles (as Newton had suggested), the two beams of light from the slits would simply form bright images of the slits on the white surface. The pattern of bright and dark bands he observed, however, is just what would be expected if light had wavelike properties. An analogy with water waves demonstrates why (Figure 5-5b).
Maxwell and Light as an Electromagnetic Wave
The discovery of the wave nature of light posed some obvious questions. What exactly is “waving” in light? That is, what is it about light that goes up and down like water waves on the ocean? Because we can see light from the Sun, planets, and stars, light waves must be able to travel across empty space. Hence, whatever is “waving” cannot be any material substance. What, then, is it?
The answer came from a seemingly unlikely source—a comprehensive theory that described electricity and magnetism. Numerous experiments during the first half of the nineteenth century demonstrated an intimate connection between electric and magnetic forces. A central idea to emerge from these experiments is the concept of a field, an immaterial yet measurable disturbance of any region of space in which electric or magnetic forces are felt. Thus, an electric charge is surrounded by an electric field, and a magnet is surrounded by a magnetic field. Experiments in the early 1800s demonstrated that moving an electric charge produces a magnetic field; conversely, moving a magnet gives rise to an electric field.
108
In the 1860s, the Scottish mathematician and physicist James Clerk Maxwell succeeded in describing all the basic properties of electricity and magnetism in four equations. This mathematical achievement demonstrated that electric and magnetic forces are really two aspects of the same phenomenon, which we now call electromagnetism.
109
By combining his four equations, Maxwell showed that electric and magnetic fields should travel through space in the form of waves at a speed of 3.0 × 105 km/s—a value equal to the best available value for the speed of light. Maxwell’s suggestion that these waves do exist and are observed as light was soon confirmed by experiments. Because of its electric and magnetic properties, light is also called electromagnetic radiation.
CAUTION!
You may associate the term radiation with radioactive materials like uranium, but this term refers to anything that radiates, or spreads away, from its source. For example, scientists sometimes refer to sound waves as “acoustic radiation”. Radiation does not have to be related to radioactivity!
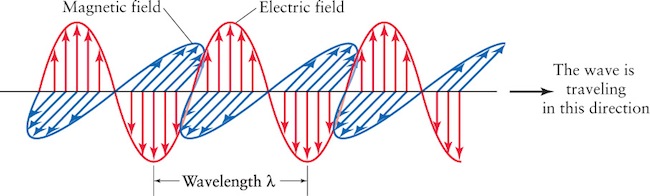
Electromagnetic radiation consists of oscillating electric and magnetic fields, as shown in Figure 5-6. The distance between two successive wave crests is called the wavelength of the light, usually designated by the Greek letter λ (lambda). It is essential to keep in mind that no matter what the wavelength, all electromagnetic radiation travels at the same speed in a vacuum: c = 3.0 × 105 km/s = 3.0 × 108 m/s.
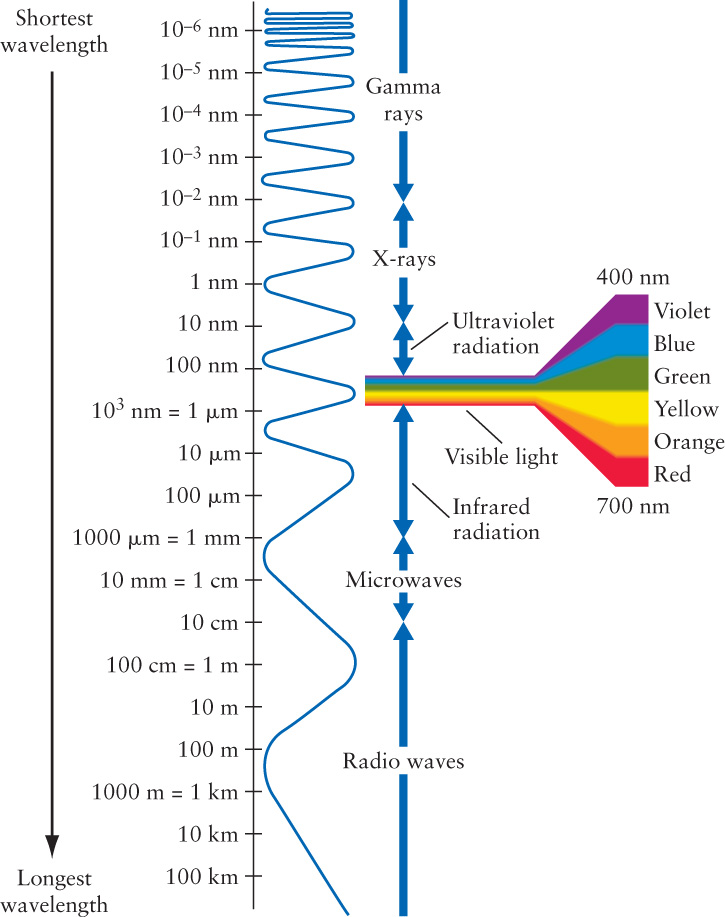
More than a century elapsed between Newton’s experiments with a prism and the confirmation of the wave nature of light. One reason for this delay is that visible light, the light to which the human eye is sensitive, has extremely short wavelengths—less than a thousandth of a millimeter—that are not easily detectable. To express such tiny distances conveniently, scientists use a unit of length called the nanometer (abbreviated nm), where 1 nm = 10−9 m. Experiments demonstrated that visible light has wavelengths covering the range from about 400 nm for violet light to about 700 nm for red light. Intermediate colors of the rainbow like yellow (550 nm) have intermediate wavelengths, as shown in Figure 5-7. (Some astronomers prefer to measure wavelengths in angstroms. One angstrom, abbreviated Å, is one-tenth of a nanometer: 1 Å = 0.1 nm = 10−10 m. In these units, the wavelengths of visible light extend from about 4000 Å to about 7000 Å. We will not use the angstrom unit in this book, however.)
110
Visible and Nonvisible Light
Maxwell’s equations place no restrictions on the wavelength of electromagnetic radiation. Hence, electromagnetic waves could and should exist with wavelengths both longer and shorter than the 400- to 700-nm range of visible light. Consequently, researchers began to look for invisible forms of light. These are forms of electromagnetic radiation to which the cells of the human retina do not respond.
The first kind of invisible radiation to be discovered actually preceded Maxwell’s work by more than a half century. Around 1800 the British astronomer William Herschel passed sunlight through a prism and held a thermometer just beyond the red end of the visible spectrum. The thermometer registered a temperature increase, indicating that it was being exposed to an invisible form of energy. This invisible energy, now called infrared radiation, was later realized to be electromagnetic radiation with wavelengths somewhat longer than those of visible light.
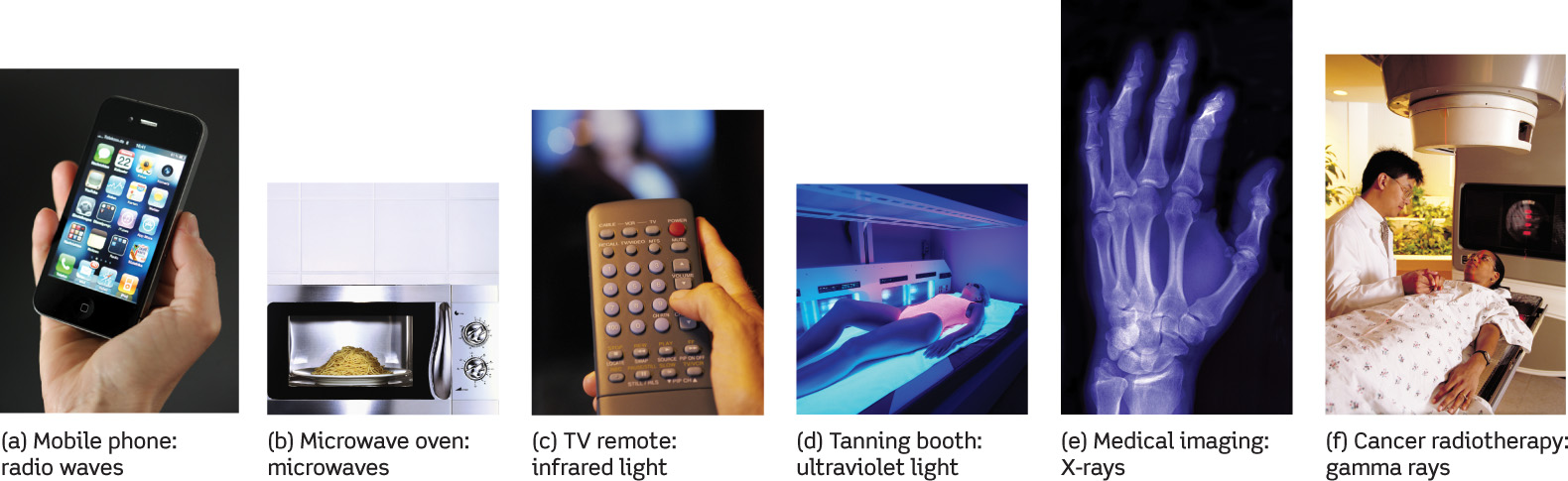
In experiments with electric sparks in 1888, the German physicist Heinrich Hertz succeeded in producing electromagnetic radiation with even longer wavelengths of a few centimeters or more. These are now known as radio waves. In 1895 another German physicist, Wilhelm Röntgen, invented a machine that produces electromagnetic radiation with wavelengths shorter than 10 nm, now known as X-rays. The X-ray machines in modern medical and dental offices are direct descendants of Röntgen’s invention. Over the years radiation has been discovered with many other wavelengths.
Thus, visible light occupies only a tiny fraction of the full range of possible wavelengths, collectively called the electromagnetic spectrum. As Figure 5-7 shows, the electromagnetic spectrum stretches from the longest-wavelength radio waves to the shortest-wavelength gamma rays. Figure 5-8 shows some applications of nonvisible light in modern technology.
On the long-wavelength side of the visible spectrum, infrared radiation covers the range from about 700 nm to 1 mm. Astronomers interested in infrared radiation often express wavelength in micrometers or microns, abbreviated μm, where 1 μm = 10−3 mm = 10−6 m. Microwaves have wavelengths from roughly 1 mm to 10 cm, while radio waves have even longer wavelengths.
At wavelengths shorter than those of visible light, ultraviolet radiation extends from about 400 nm down to 10 nm. Next are X-rays, which have wavelengths between about 10 and 0.01 nm, and beyond them at even shorter wavelengths are gamma rays. Note that the rough boundaries between different types of radiation are simply arbitrary divisions in the electromagnetic spectrum.
Once again, in the vacuum of space, all light—from radio waves to gamma rays—travels at the speed of light, c = 3 × 108 m/s.
111
CONCEPT CHECK 5-3
Which form of electromagnetic radiation has a wavelength similar to the diameter of your finger?
Frequency and Wavelength
Astronomers who work with radio telescopes often prefer to speak of frequency rather than wavelength. The frequency of a wave is the number of wave crests that pass a given point in one second. Equivalently, it is the number of complete cycles of the wave that pass per second (a complete cycle is from one crest to the next). Frequency is usually denoted by the Greek letter ν (nu). The unit of frequency is the cycle per second, also called the hertz (abbreviated Hz) in honor of Heinrich Hertz, the physicist who first produced radio waves. For example, if 500 crests of a wave pass you in one second, the frequency of the wave is 500 cycles per second or 500 Hz.
In working with frequencies, it is often convenient to use the prefix mega- (meaning “million,” or 106, and abbreviated M) or kilo- (meaning “thousand,” or 103, and abbreviated k). For example, AM radio stations broadcast at frequencies between 535 and 1605 kHz (kilohertz), while FM radio stations broadcast at frequencies in the range from 88 to 108 MHz (megahertz).
The relationship between the frequency and wavelength of an electromagnetic wave is a simple one. Because light moves at a constant speed c = 3 × 108 m/s, if the wavelength (distance from one crest to the next) is made shorter, the frequency must increase (more of those closely spaced crests pass you each second). Thus, the shorter the wavelength of light, the higher the frequency, and likewise, the longer the wavelength of light, the lower the frequency. The frequency ν of light is related to its wavelength λ by the equation
Frequency and wavelength of an electromagnetic wave
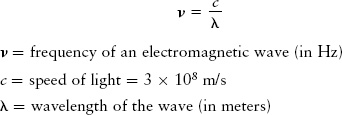
That is, the frequency of a wave equals the wave speed divided by the wavelength.
For example, hydrogen atoms in space emit radio waves with a wavelength of 21.12 cm. To calculate the frequency of this radiation, we must first express the wavelength in meters rather than centimeters: λ = 0.2112 m. Then we can use the above formula to find the frequency ν:
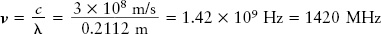
Visible light has a much shorter wavelength and higher frequency than radio waves. You can use the above formula to show that for yellow-orange light of wavelength 600 nm, the frequency is 5 × 1014 Hz or 500 million megahertz!
While Young’s experiment (Figure 5-5) showed convincingly that light has wavelike aspects, it was discovered in the early 1900s that light also has some of the characteristics of a stream of particles and waves. We will explore light’s dual nature in Section 5-5.
112
CONCEPT CHECK 5-4
How do the frequencies of the longest wavelengths of light compare to the frequencies of the shortest wavelengths of light?
CALCULATION CHECK 5-1
What is the wavelength of radio waves for the FM radio station 99.9 KTYD (which is 99.9 MHz)?