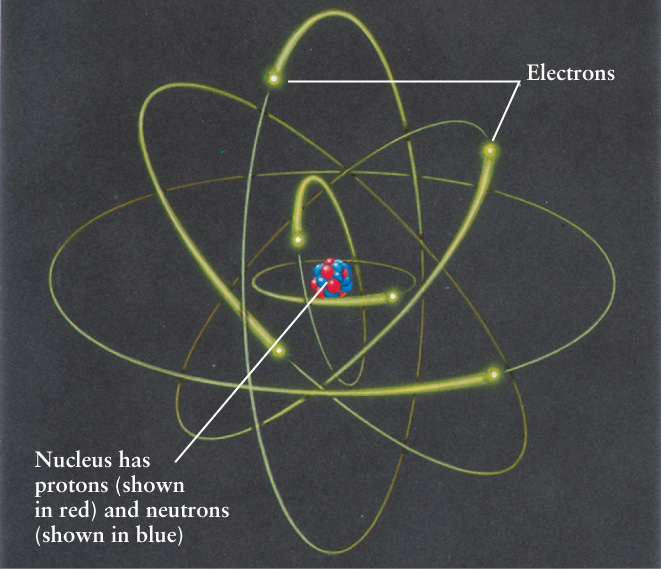
5-7 An atom consists of a small, dense nucleus surrounded by electrons
To decode the information in the light from immense objects like stars and galaxies, we must understand the structure of atoms
By the early twentieth century, it was known that atoms contain negatively charged particles called electrons and some material with positive charge, but the structure of atoms was still a mystery. The “plum pudding” model popular at that time proposed that the negative electrons were like plums uniformly mixed into a positively charged pudding. However, in 1910 this model was overturned by Ernest Rutherford, a gifted physicist from New Zealand.
The Nucleus of an Atom
Rutherford discovered a more accurate model for the structure of an atom, shown in Figure 5-20. According to this model, a massive, positively charged nucleus at the center of the atom is orbited by tiny, negatively charged electrons. Rutherford concluded that at least 99.98% of the mass of an atom must be concentrated in its nucleus, whose diameter is only about 10−14 m. (The diameter of a typical atom is far larger, about 10−10 m.)
ANALOGY
To appreciate just how tiny the nucleus is, imagine expanding an atom by a factor of 1012 to a diameter of 100 meters, about the length of a football field. On this scale, the nucleus would be just a centimeter across (no larger than your thumbnail) in the middle of the field, and the electrons would be orbiting at a distance near the goal posts.
We know today that the nucleus of an atom contains two types of particles, protons and neutrons. A proton has a positive electric charge, equal in magnitude to that of the negatively charged electron. As its name suggests, a neutron has no electric charge—it is electrically neutral. As an example, the helium atom has two protons and two neutrons. Protons and neutrons are held together in a nucleus by the so-called strong nuclear force, whose great strength overcomes the electric repulsion between the positively charged protons. A proton and a neutron have almost the same mass, 1.7 × 10−27 kg, and each has about 2000 times as much mass as an electron (9.1 × 10−31 kg). In an ordinary atom there are as many positive protons as there are negative electrons, so the atom has no net electric charge. Because the mass of the electron is so small, the mass of an atom is not much greater than the mass of its nucleus.
TOOLS OF THE ASTRONOMER’S TRADE
Atoms, the Periodic Table, and Isotopes
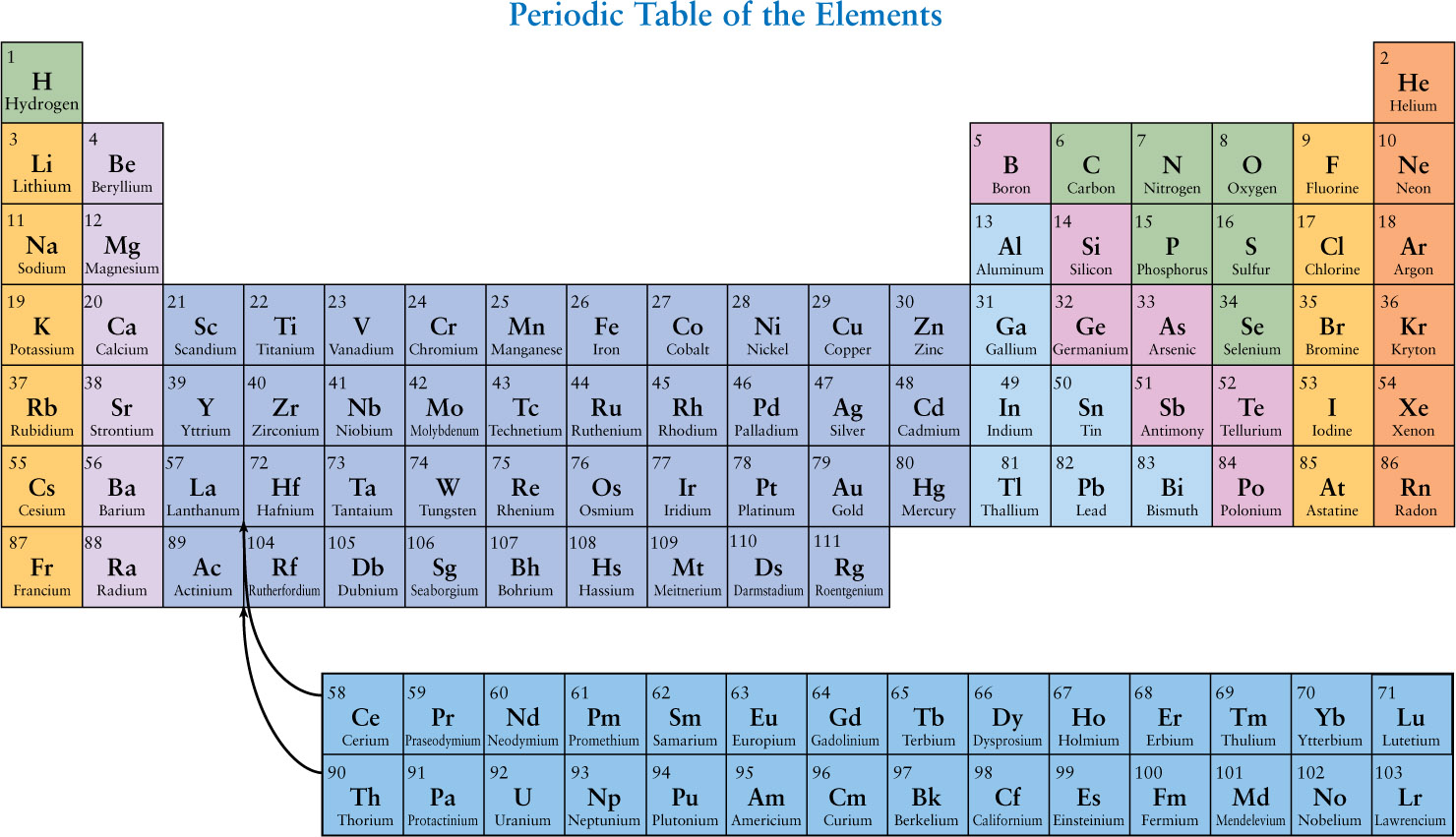
Each different chemical element is made of a specific type of atom. Each specific atom has a characteristic number of protons in its nucleus. For example, a hydrogen atom has 1 proton in its nucleus, an oxygen atom has 8 protons in its nucleus, and so on.
The number of protons in an atom’s nucleus is the atomic number for that particular element. The chemical elements are most conveniently listed in the form of a periodic table (shown in the figure). Elements are arranged in the periodic table in order of increasing atomic number. With only a few exceptions, this sequence also corresponds to increasing average mass of the atoms of the elements. Thus, hydrogen (symbol H), with atomic number 1, is the lightest element. Iron (symbol Fe) has atomic number 26 and is a relatively heavy element.
All the elements listed in a single vertical column of the periodic table have similar chemical properties. For example, the elements in the far right column are all gases under the conditions of temperature and pressure found at Earth’s surface, and they are all very reluctant to react chemically with other elements.
In addition to nearly 100 naturally occurring elements, the periodic table includes a number of artificially produced elements. Most of these elements are heavier than uranium (symbol U) and are highly radioactive, which means that they decay into lighter elements within a short time of being created in laboratory experiments. Scientists have succeeded in creating only a few atoms of elements 104 and above.
The number of protons in the nucleus of an atom determines which element that atom is. Nevertheless, the same element may have different numbers of neutrons in its nucleus. For example, oxygen (O) has atomic number 8, so every oxygen nucleus has exactly 8 protons. But oxygen nuclei can have 8, 9, or 10 neutrons. These three slightly different kinds of oxygen are called isotopes. The isotope with 8 neutrons is by far the most abundant variety. It is written as 16O, or oxygen-16. The rarer isotopes with 9 and 10 neutrons are designated as 17O and 18O, respectively.
The superscript that precedes the chemical symbol for an element equals the total number of protons and neutrons in a nucleus of that particular isotope. For example, a nucleus of the most common isotope of iron, 56Fe or iron-56, contains a total of 56 protons and neutrons. From the periodic table, the atomic number of iron is 26, so every iron atom has 26 protons in its nucleus. Therefore, the number of neutrons in an iron-56 nucleus is 56 − 26 = 30. (Most nuclei have more neutrons than protons, especially in the case of the heaviest elements.)
It is extremely difficult to distinguish chemically between the various isotopes of a particular element. Ordinary chemical reactions involve only the electrons that orbit the atom, never the neutrons buried in its nucleus. But there are small differences in the wavelengths of the spectral lines for different isotopes of the same element. For example, the spectral line wavelengths of the hydrogen isotope 2H are about 0.03% greater than the wavelengths for the most common hydrogen isotope, 1H. Thus, different isotopes can be distinguished by careful spectroscopic analysis.
Isotopes are important in astronomy for a variety of reasons. By measuring the relative amounts of different isotopes of a given element in a Moon rock or meteorite, the age of that sample can be determined. The mixture of isotopes left behind when a star explodes into a supernova (see Section 1-3) tells astronomers about the processes that led to the explosion. And knowing the properties of different isotopes of hydrogen and helium is crucial to understanding the nuclear reactions that make the Sun shine. Look for these and other applications of the idea of isotopes in later chapters.
127
While the solar system is held together by gravitational forces, electrons are held in atoms by electrical forces. Opposites attract: The negative charges on the orbiting electrons are attracted to the positive charges on the protons in the nucleus. Box 5-5 describes more about the connection between the structure of atoms and the chemical and physical properties of substances made of those atoms.
128
Rutherford’s new model clarified the structure of the atom, but did not explain how these tiny particles within the atom give rise to spectral lines. The task of reconciling Rutherford’s atomic model with Kirchhoff’s laws of spectral analysis was undertaken by the young Danish physicist Niels Bohr, who joined Rutherford’s laboratory in 1912.