Chapter 2. Analyzing Enzymes and Enzyme Kinetics I
Exercise 1. The Enzymatic Oxidation of Catechol—Reaction Rate
General Purpose
Conceptual
- Understand the general model of enzyme catalysis.
- Understand the relationship between light absorption and the concentration of a substance.
- Understand how to use a standard curve for the determination of an unknown.
- Understand the way data should be presented in table form.
- Understand the structure of a graph.
- Understand how to make a graph that represents data.
Procedural
- Gain proficiency in the use of pipettes and pipetting.
- Gain proficiency in the use of a spectrometer.
Materials per Student
Disposable gloves
Safety glasses
Materials per Student Group (2 students)
Spectrometer
Test tube rack
Small Parafilm squares
Deionized water (dH2O)
Potato extract
0.05 M Catechol
One 10-mL pipette and green pi-pump
4 cuvettes
p 1000 micropipette
Procedure
Part I - Spectrometer set-up and calibration
1. Turn on the spectrometer by holding the power button on the right side for about three seconds or until the LED lights start to flash (see Figure 2-12).
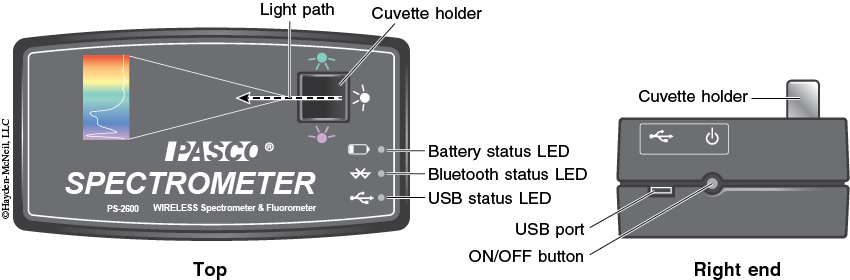
2. Plug the spectrometer into the computer via the USB cable (see Figure 2-12). To wirelessly connect to the spectrometer with a personal laptop or tablet, add a device in the Bluetooth settings and select the spectrometer that matches with the ID # on the bottom of the instrument.
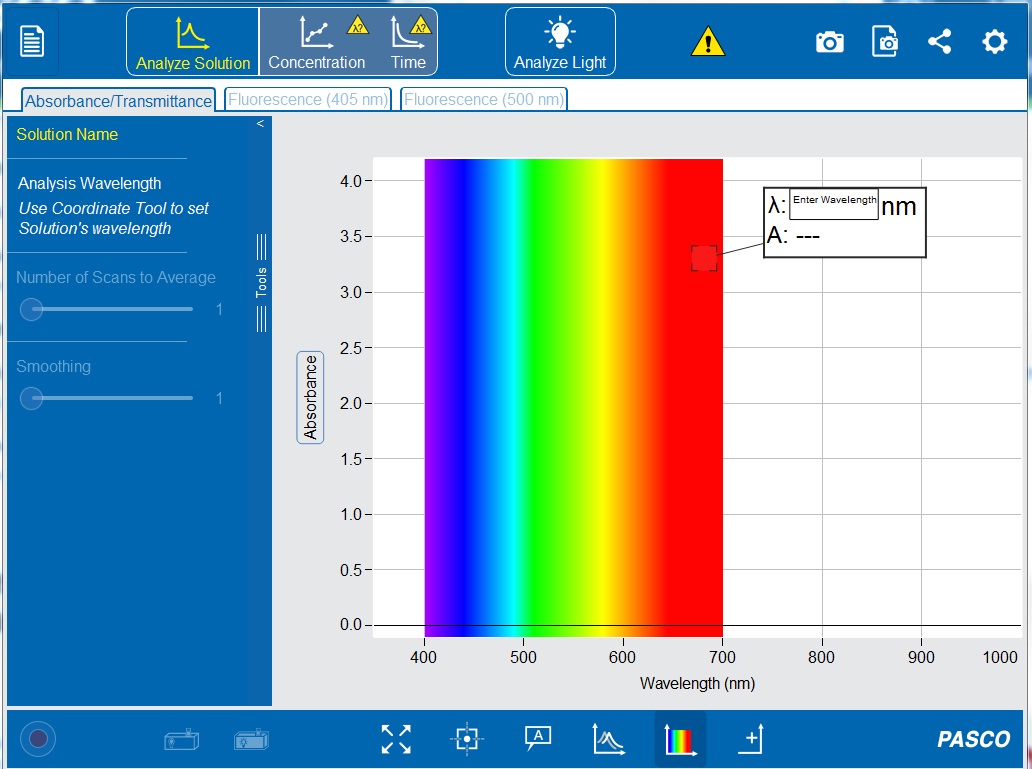
3. Open the PASCO Spectrometry app by clicking the icon ![]() . This should now show the initial screen of the PASCO Spectrometry display (see Figure 2-13) on your computer or tablet.
. This should now show the initial screen of the PASCO Spectrometry display (see Figure 2-13) on your computer or tablet.
4. After a few seconds, the ‘Connection Status’ symbols on the top toolbar should turn from ![]() to
to ![]() . Click the circle to check that the ID # on the bottom of the spectrometer matches the display on your computer or tablet. If connecting wirelessly, a ‘Sensor Not Found’ error should pop up. Click
. Click the circle to check that the ID # on the bottom of the spectrometer matches the display on your computer or tablet. If connecting wirelessly, a ‘Sensor Not Found’ error should pop up. Click ![]() . Select the spectrometer with the corresponding ID # and click
. Select the spectrometer with the corresponding ID # and click ![]() . If a connection error does not pop up, click
. If a connection error does not pop up, click ![]() on the top toolbar.
on the top toolbar.
5. Make sure the “Absorbance/Transmittance” tab ![]() is open on the top toolbar and the “Analyze Solution”
is open on the top toolbar and the “Analyze Solution” ![]() setting is selected.
setting is selected.
6. Obtain a tube of potato extract from your instructor. Cap the tube to limit oxygen exposure and place it on ice.
7. Obtain a clean cuvette from the rack. Using the labelled pipettes, prepare a blank based on the solutions and volumes listed in Table 2-1.

Be sure to recap the large tube containing potato extract to limit oxygen exposure and return it to the ice bucket.
8. With gloves on, use your thumb or forefinger to seal the blank cuvette and invert it once. Wipe the blank cuvette with a Kimwipe and gently insert it into the cuvette holder. The cuvette should fit snugly.
9. On the bottom toolbar, click “Calibrate Dark” ![]() to calibrate the spectrometer with the light turned off. A progress bar should appear, and after the calibration is complete, a check mark
to calibrate the spectrometer with the light turned off. A progress bar should appear, and after the calibration is complete, a check mark ![]() should appear.
should appear.
10. Wait one to two minutes to allow the light source to warm up, then click “Calibrate Reference” ![]() to calibrate the spectrometer to the blank solution with the light on.
to calibrate the spectrometer to the blank solution with the light on.
11. On the bottom toolbar, click “Start Record” ![]() . The symbol will change to the “Stop Record”
. The symbol will change to the “Stop Record” ![]() icon and a horizontal line should appear at 0.0 ODU, indicating the spectrometer was properly calibrated. Tiny peaks in the line show noise, but if there are major peaks, repeat steps 9 and 10 to recalibrate the spectrometer.
icon and a horizontal line should appear at 0.0 ODU, indicating the spectrometer was properly calibrated. Tiny peaks in the line show noise, but if there are major peaks, repeat steps 9 and 10 to recalibrate the spectrometer.
12. In the “Enter Wavelength” box on the display (see Figure 2-13) type 500 nm to set the wavelength to 500 nanometers. If the white Enter Wavelength box is not already displayed on the graph, click the “Add Coordinate Tool” ![]() on the bottom toolbar. The coordinate tool can also be manually moved to set a wavelength.
on the bottom toolbar. The coordinate tool can also be manually moved to set a wavelength.
13. Click ![]() to set the wavelength. A vertical line should appear on the graph at 500 nm, and the white box should now display the wavelength (λ) and the absorbance value (A).
to set the wavelength. A vertical line should appear on the graph at 500 nm, and the white box should now display the wavelength (λ) and the absorbance value (A).
14. Remove the blank cuvette from the spectrometer and return it to the rack.
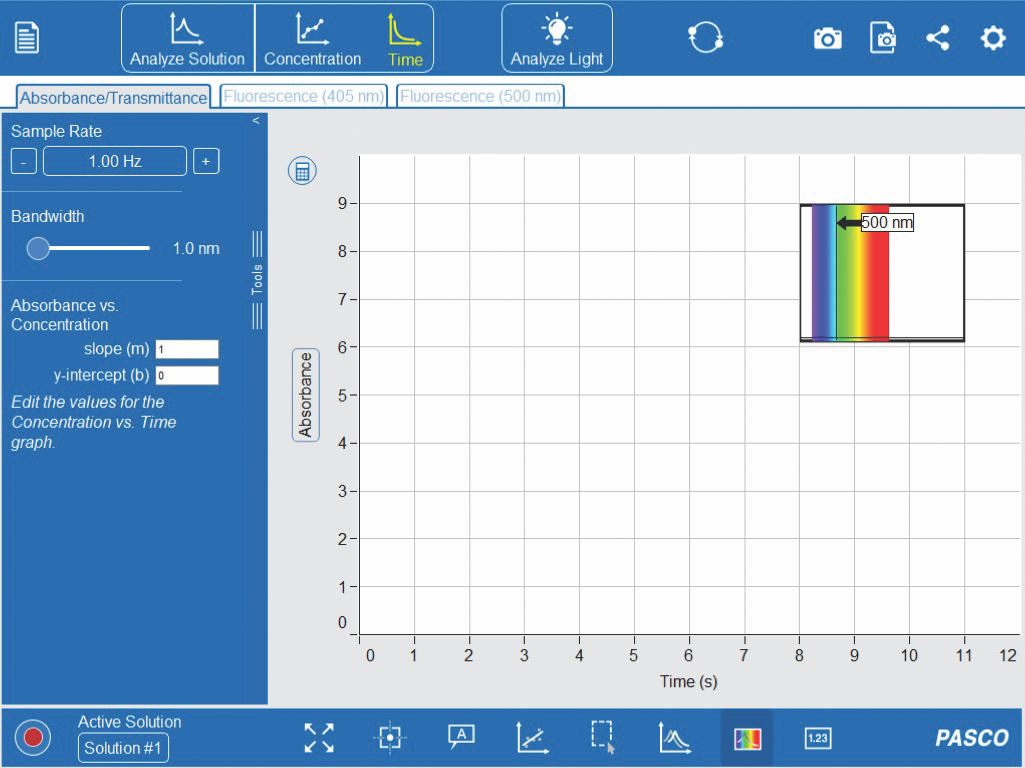
15. As seen in Figure 2-14, click the ‘Time’ graph on the top toolbar. Keep the default options on the left-hand toolbar. A small absorbance spectrum from the ‘Analyze Solution’ tab should be visible as well.
Part II - Preparing the Cuvettes for Treatment 1
1. Obtain three clean cuvettes from the rack. Using the labelled pipettes, prepare three replicate treatment cuvettes based on the solutions and volumes listed in Table 2-1.
Part III - Measuring the Reaction Rate
1. To start the reaction, add the volume of potato extract listed in Table 2-1 to the first replicate using the labelled pipette.
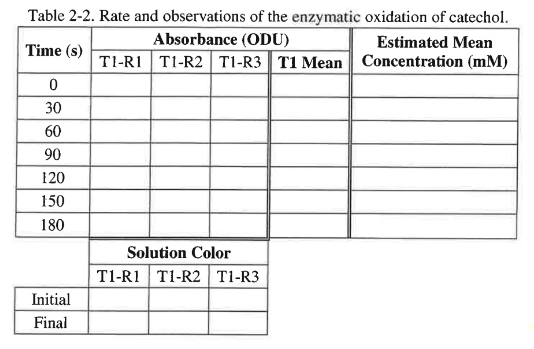
2. Quickly seal and invert the cuvette once to mix the contents. Make note of the color of the solution in Table 2-2.
3. Quickly wipe the cuvette with a Kimwipe, place it in the cuvette holder, and click ‘Record’ to start measuring absorbance over time.
4. After 180 seconds, click ‘Record’ again to stop recording data.
5. Remove the cuvette and record the final colors of the solution in Table 2-2.
6. Repeat steps 1-5 for the second and third replicates. You may need to calibrate with the blank between replicates.
Part IV - Calculations
1. For each replicate/run graph, use the coordinate tool on the bottom toolbar to obtain the absorbance values at the times needed for Table 2-2 by manually moving the ‘Coordinate Tool’ along the graphs.
2. Calculate the mean absorbance of the 3 replicates at each time point for Treatment 1 and record the values in Table 2-2.
3. Use the standard curve and a ruler to estimate concentrations from mean of the absorbance reading you had calculated. Record the estimated concentrations values in Table 2-2.
Clean-Up Procedure
1. Turn off the spectrometer by holding the power button.
2. Close the PASCO Spectrometry app. Leave the lab computer on.
3. With gloves and safety glasses on, drain and rinse all the cuvettes in the sink.
4. Return the four cuvettes to the rack on the bench paper upside down to drain.
5. Rinse and return the large tube to the rack near the sink upside down to drain.
6. Return the pi-pumps to the basket. Return the rulers and labelled pipettes to the appropriate jars.
7. Discard (only) gloves in the biohazard bag.
8. Return the safety glasses, protocol sheet, and standard curve to the front table.
9. Push in your chair. See your instructor regarding any In-Class Assignment.
Determination of the Reaction Rate
Reaction rates are often expressed as changes in the concentration of product over time (e.g., millimoles of product/minute). The observations you have made during this exercise measure light absorption by the reaction solution. This light absorption is based on the concentration of chemicals in the solution (e.g., millimoles of substance/liter). To convert the absorption readings (ODU) to values related to the concentration of product you will use a standard curve (located on your lab bench) that shows the relationship between absorption and benzoquinone concentration.
Determine the concentration of benzoquinone for each of your observations and enter that information in the table you created in your lab notebook. Using the concentration data, on a new page in your laboratory notebook, create a graph of product (benzoquinone) concentration versus time. Include a figure caption and if needed a legend. Determine if the points (or some of the points) you plotted represent a straight line and if they do then add a best-fit trendline to your graph. The slope of this trendline (increase in product concentration/unit time) is directly related to the reaction rate (increase in product/unit time) for your reaction. Your graph will be evaluated using the rubric provided by your laboratory instructor. Make sure that you have evaluated your graph before submitting it to your instructor.
Preparation for Specific Enzyme Analysis
For the second enzymes lab, your lab group will design an experiment to investigate the impact of one of the following treatments on the basic enzymatic reaction you just studied (i.e., oxidation of catechol catalyzed by catechol oxidase).
The possible treatments are:
- Enzyme concentration
- pH
- Substrate concentration
- Temperature
- Structure of the substrate
- Source of enzyme
Your lab instructor will describe the general aspects of these various treatments and then your group will decide which of the factors above you want to investigate. If your group selects source of enzyme then you will need to obtain the material before the next lab meeting. You will tell your lab instructor which treatment your group has chosen and record your choice in your lab notebook.
Once your group has chosen the treatment for the next lab the next step is to make a prediction about how changing that factor might affect the rate of the reaction. Your prediction should be based on your understanding of how enzymes catalyze chemical reactions. If you have trouble with this, ask your laboratory instructor for help. The predictions are part of the scientific method (Figure 2-15).
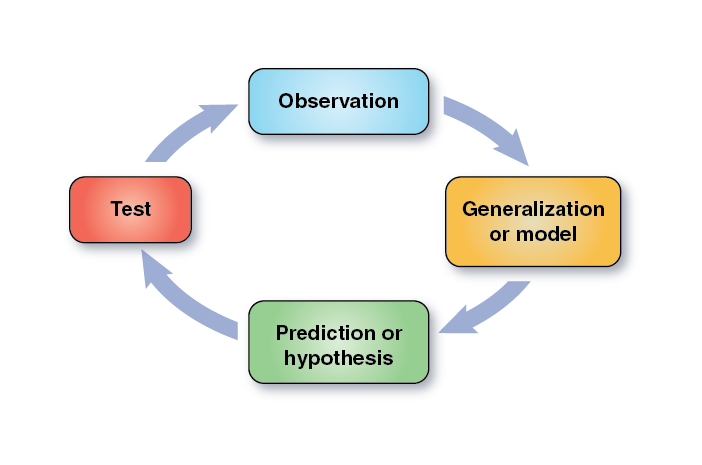
Your prediction can now be stated as a set of formal hypotheses. Hypothesis formation is one of the most important and often the most difficult steps of the scientific method. One important consideration in the formulation of testable hypotheses is to be sure that the data will allow one of the hypotheses to be rejected, thereby allowing the other hypothesis to be supported. In order to ensure that this happens, researchers often state their hypotheses in the form of a null hypothesis (H0) and an alternative hypothesis (Ha). The null hypothesis states that your treatment will have no effect on the process being studied. In other words there will be no meaningful difference in the results between the control group and the experimental or between various treatment groups. The alternative hypothesis is the alternative to the null hypothesis and states that there will be some effect based on the treatment involved. This means there should be some meaningful difference in the results between the control group and the experimental or between various treatment groups. The overall objective is to set up hypotheses which will result in one or the other being rejected (falsified) at the end of the experiment. Science only proceeds when a hypothesis is rejected. Remember in science you can only disprove something, never prove it.
As a group, formulate a null hypothesis and an alternative hypothesis for the next experiment based on the treatment your group has chosen. Before leaving lab make sure your lab instructor knows what your hypotheses are for the next experiment.
Post-Lab Quiz
Proceed to the Post-Lab Quiz