Chapter 2. Carbon Metabolism: Light and Photosynthetic Pigments
General Purpose
This pre-lab will present some of the characteristics of light and the general concepts related to light absorption, transmittance, and fluorescence.
Learning Objectives
General Purpose
Conceptual
- Gain an understanding of the relationship between absorbance and transmittance of light and photosynthetic pigments.
- Know what fluorescence is and how it is associated with the absorption of light by chlorophyll.
Background Information
White light is a continuum of wavelengths. It is composed of all the wavelengths (colors) in the spectrum of visible light. These wavelengths are measured in nanometers (nm) and range from approximately 380 to 750 nm. It is this part of the electromagnetic spectrum that drives the photosynthetic process.
When light strikes an object, it may be reflected, transmitted, or absorbed. Substances that absorb light are called pigments. Pigments selectively absorb certain wavelengths of visible light and reflect or transmit others. Therefore an object’s color is composed only of those wavelengths of light reflected or transmitted. For example, a leaf appears green because it reflects and transmits green wavelengths but absorbs all other wavelengths (colors) of light.
The photosynthetic pigments are located in the thylakoid membranes of the chloroplasts. Here they absorb light, become chemically excited, and pass their electrons to the electron carriers of Photosystem I and Photosystem II (Figure 7-5).
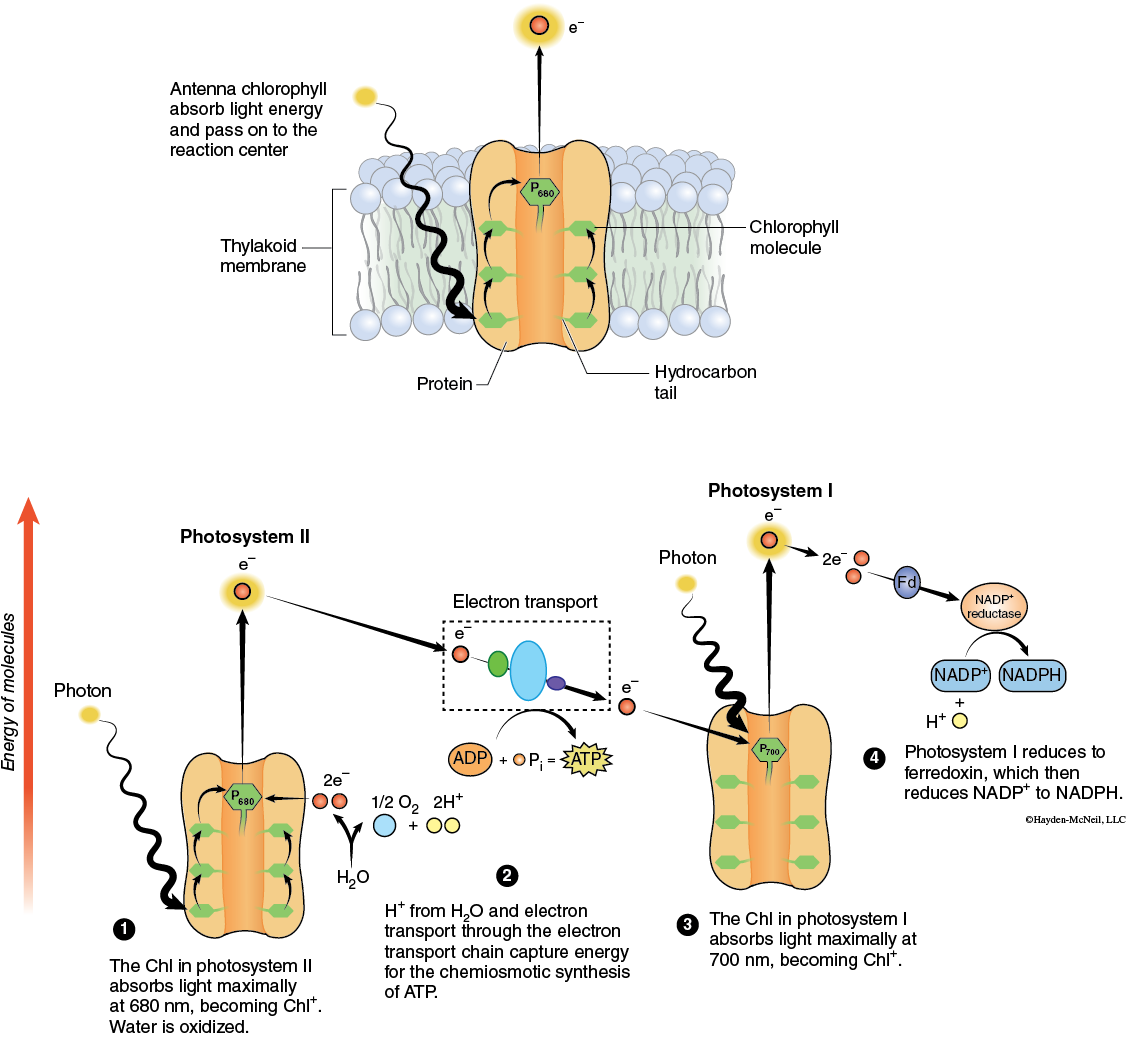
When a chlorophyll molecule absorbs light energy the excite electron is normally passed on to the molecules in the electron transport chain. However, when chlorophyll is extracted for the membrane the excited electron can no longer be passed to the electron transport chain and it loses its energy. In doing so the light that was absorbed is re-emitted as light of a longer wavelength (red). The phenomenon is known as fluorescence. Under light-saturating conditions, fluorescence can occur in intact leaves, although it is not easily observable and can only be detected with appropriate instruments.
In order for the energy in light to be used to power the reactions that occur in photosynthesis the energy of the light must be absorbed. The pigments present in chloroplasts of spinach leaves and Spirulina (a cyanobacterium) absorb light of different wavelengths. Light of different wavelengths contains different amounts of energy. This means that spinach leaves and Spirulina can make use of a wide range of light energy.
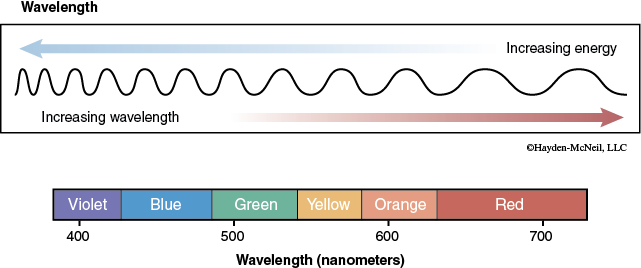
Determining what wavelengths of light the chloroplast pigments absorb can help to determine which wavelengths of light may be most useful in the process of photosynthesis.
Pre-Lab Quiz
Proceed to the Pre-Lab Quiz