Chapter 2. Carbon Metabolism: Rate of Photosynthesis and Cellular Respiration
General Purpose
This pre-lab will present some of the general concepts related to how and why CO2 levels in solution effect the pH of the solution.
Learning Objectives
General Purpose
Conceptual
- Understand the relationship between CO2 and the pH of the solution.
Procedural
- Understand basic use of a pH indicator.
Background Information
The net rate of CO2 production or consumption will be measured indirectly by monitoring pH changes of the solutions that occur because of change in the levels of CO2 . To understand why the level of CO2 in a solution has an impact on the pH of the solution requires an examination of two chemical reactions involving CO2. The first reaction involves the interaction of CO2and water (H2O) to form carbonic acid H2CO3 (Figure 8-7).
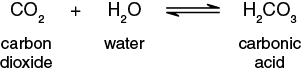
The second reaction occurs as the carbonic acid acidifies the solution. Carbonic acid is a weak acid and will dissociate in water (Figure 8-8) to form hydrogen ions (H+) and bicarbonate ions (HCO3-).
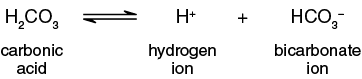
Since these are equilibrium reactions that are reversible, as CO2 is used, carbonic acid breaks down to from water and more carbon dioxide. As the carbonic acid concentration decreases, available bicarbonate associates with hydrogen ions to form more carbonic acid. The more CO2 that is used, the more H+ that is removed from the solution. As the concentration of hydrogen ions in the solution changes, so does the pH.
Chemicals known as indicators can be used to monitor pH changes in solutions. These indicators undergo a color change in solution when a pH change occurs. One such indicator is phenol red (Figure 8-9).
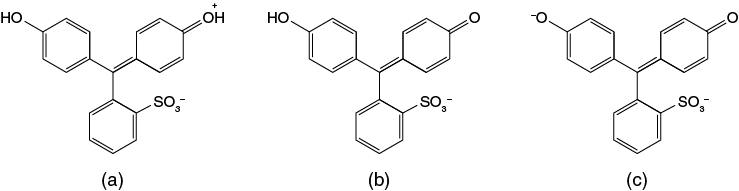
Phenol red can occur in solution in 3 different forms depending on the pH of the solution. At very acidic pHs (below pH 1.2) the maximum number of hydrogen ions (two H+ ions) are associated with its structure and the molecule has an orange-red color. Between pH1.2 and pH 7.7 the compound is yellow. Above pH 7.7 the compound becomes red. Since the change from the yellow form to the red form occurs near neutral pH it can be used to monitor changes in pH for many biological relevant reactions. The different color forms of phenol red absorb light at different wavelengths. This variation in light absorbance properties allows solution pH changes to be followed quantitatively using a spectrophotometer.
Pre-Lab Quiz
Proceed to the Pre-Lab Quiz