APPENDIX: Visualizing Molecular Structures I: Small Molecules
APPENDIX: Visualizing Molecular Structures I: Small Molecules
The authors of a biochemistry textbook face the problem of trying to present three-
Stereochemical Renderings
Most of the chemical formulas in this book are drawn to depict the geometric arrangement of atoms, crucial to chemical bonding and reactivity, as accurately as possible. For example, the carbon atom of methane is tetrahedral, with H–

To illustrate the correct stereochemistry about tetrahedral carbon atoms, wedges will be used to depict the direction of a bond into or out of the plane of the page. A solid wedge with the broad end away from the carbon atom denotes a bond coming toward the viewer out of the plane. A dashed wedge, with its broad end at the carbon atom, represents a bond going away from the viewer behind the plane of the page. The remaining two bonds are depicted as straight lines.
Fischer Projections
Although representative of the actual structure of a compound, stereochemical structures are often difficult to draw quickly. An alternative, less-
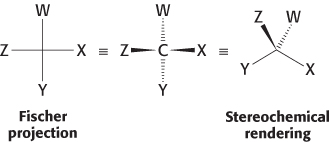
In a Fischer projection, the bonds to the central carbon are represented by horizontal and vertical lines from the substituent atoms to the carbon atom, which is assumed to be at the center of the cross. By convention, the horizontal bonds are assumed to project out of the page toward the viewer, whereas the vertical bonds are assumed to project behind the page away from the viewer.
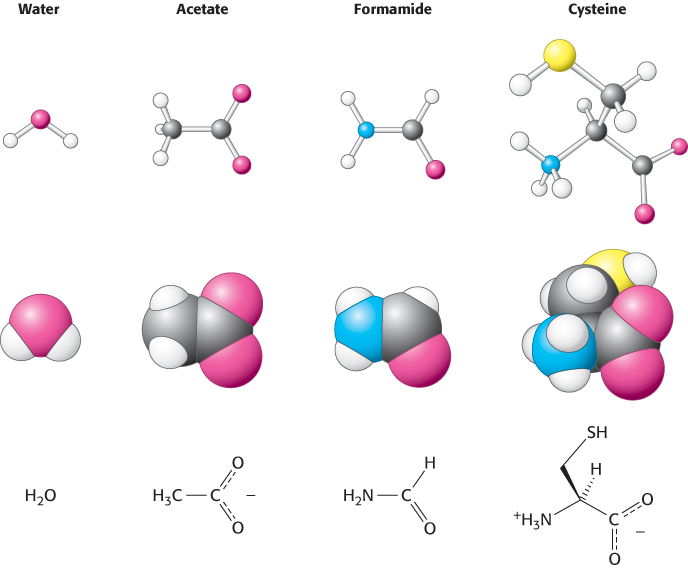
Molecular Models for Small Molecules
For depicting the molecular architecture of small molecules in more detail, two types of models will often be used: space filling and ball and stick. These models show structures at the atomic level.
1. Space-
|
Carbon, black |
Hydrogen, white |
Nitrogen, blue |
|
Oxygen, red |
Sulfur, yellow |
Phosphorus, purple |
Space-
2. Ball-
23
Molecular models for depicting large molecules will be discussed in the appendix to (Chapter 2).