Protein Composition and Structure
CHAPTER
2
27
OUTLINE
Proteins are the most versatile macromolecules in living systems and serve crucial functions in essentially all biological processes. They function as catalysts, transport and store other molecules such as oxygen, provide mechanical support and immune protection, generate movement, transmit nerve impulses, and control growth and differentiation. Indeed, much of this book will focus on understanding what proteins do and how they perform these functions.
Several key properties enable proteins to participate in a wide range of functions.
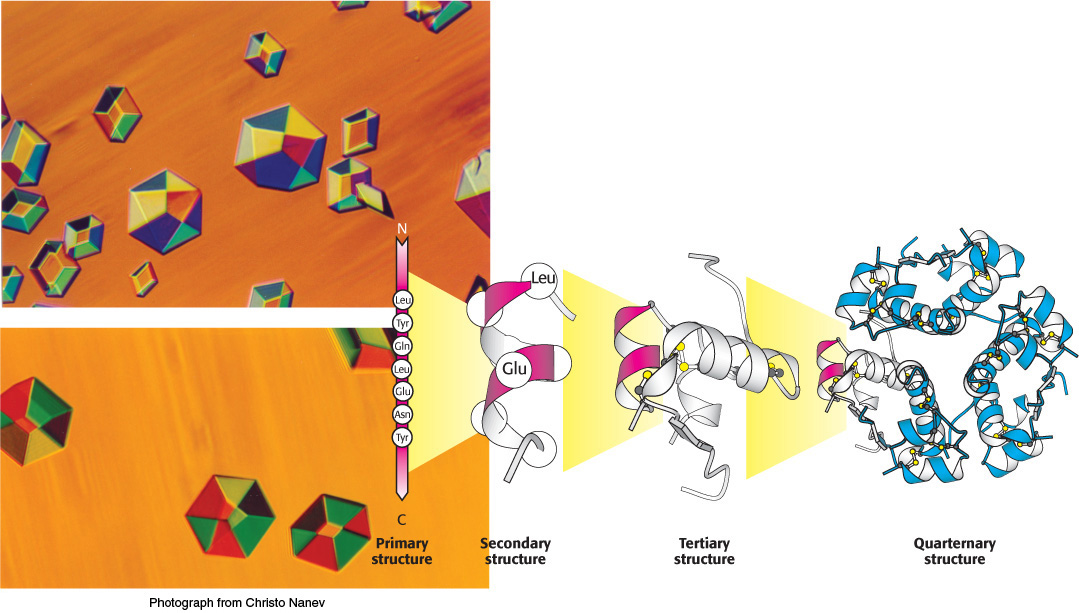
1. Proteins are linear polymers built of monomer units called amino acids, which are linked end to end. The sequence of linked amino acids is called the primary structure. Remarkably, proteins spontaneously fold up into three-
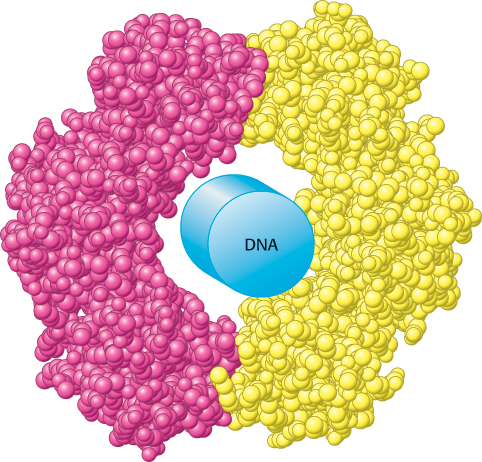
 FIGURE 2.1 Structure dictates function. A protein component of the DNA replication machinery surrounds a section of DNA double helix depicted as a cylinder. The protein, which consists of two identical subunits (shown in red and yellow), acts as a clamp that allows large segments of DNA to be copied without the replication machinery dissociating from the DNA.
FIGURE 2.1 Structure dictates function. A protein component of the DNA replication machinery surrounds a section of DNA double helix depicted as a cylinder. The protein, which consists of two identical subunits (shown in red and yellow), acts as a clamp that allows large segments of DNA to be copied without the replication machinery dissociating from the DNA.
28
2. Proteins contain a wide range of functional groups. These functional groups include alcohols, thiols, thioethers, carboxylic acids, carboxamides, and a variety of basic groups. Most of these groups are chemically reactive. When combined in various sequences, this array of functional groups accounts for the broad spectrum of protein function. For instance, their reactive properties are essential to the function of enzymes, the proteins that catalyze specific chemical reactions in biological systems (Chapters 8 through 10).
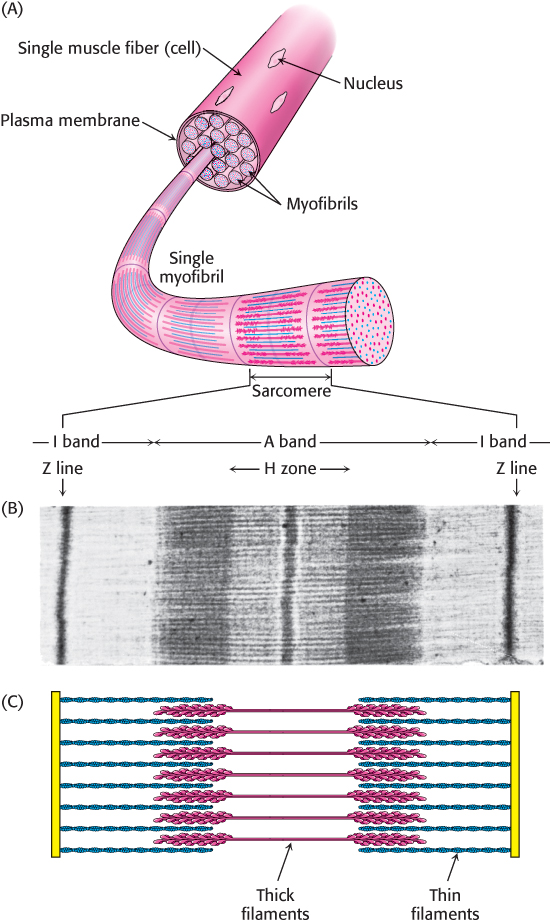
3. Proteins can interact with one another and with other biological macromolecules to form complex assemblies. The proteins within these assemblies can act synergistically to generate capabilities that individual proteins may lack. Examples of these assemblies include macromolecular machines that replicate DNA, transmit signals within cells, and enable muscle cells to contract (Figure 2.2).
29
4. Some proteins are quite rigid, whereas others display considerable flexibility. Rigid units can function as structural elements in the cytoskeleton (the internal scaffolding within cells) or in connective tissue. Proteins with some flexibility may act as hinges, springs, or levers. In addition, conformational changes within proteins enable the regulated assembly of larger protein complexes as well as the transmission of information within and between cells (Figure 2.3).
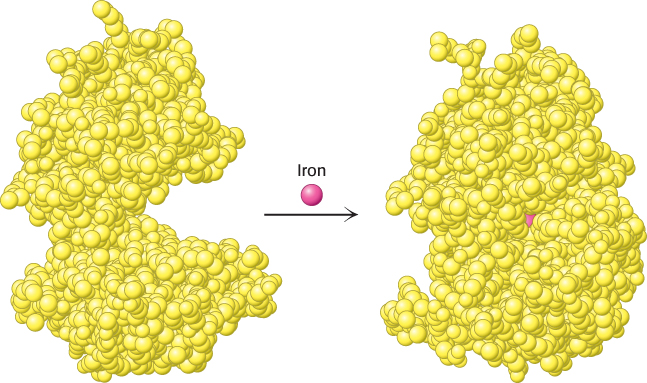
 FIGURE 2.3 Flexibility and function. On binding iron, the protein lactoferrin undergoes a substantial change in conformation that allows other molecules to distinguish between the iron-
FIGURE 2.3 Flexibility and function. On binding iron, the protein lactoferrin undergoes a substantial change in conformation that allows other molecules to distinguish between the iron-