2.1Proteins Are Built from a Repertoire of 20 Amino Acids
Proteins Are Built from a Repertoire of 20 Amino Acids
Amino acids are the building blocks of proteins. An α-amino acid consists of a central carbon atom, called the α carbon, linked to an amino group, a carboxylic acid group, a hydrogen atom, and a distinctive R group. The R group is often referred to as the side chain. With four different groups connected to the tetrahedral α-carbon atom, α-amino acids are chiral: they may exist in one or the other of two mirror-
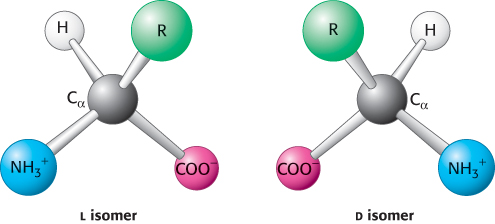
Notation for distinguishing stereoisomers
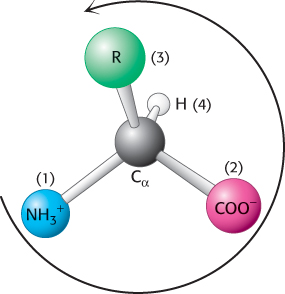
The four different substituents of an asymmetric carbon atom are assigned a priority according to atomic number. The lowest-
 Only l amino acids are constituents of proteins. For almost all amino acids, the l isomer has S (rather than R) absolute configuration (Figure 2.5). What is the basis for the preference for l amino acids? The answer has been lost to evolutionary history. It is possible that the preference for l over d amino acids was a consequence of a chance selection. However, there is evidence that l amino acids are slightly more soluble than a racemic mixture of d and l amino acids, which tend to form crystals. This small solubility difference could have been amplified over time so that the l isomer became dominant in solution.
Only l amino acids are constituents of proteins. For almost all amino acids, the l isomer has S (rather than R) absolute configuration (Figure 2.5). What is the basis for the preference for l amino acids? The answer has been lost to evolutionary history. It is possible that the preference for l over d amino acids was a consequence of a chance selection. However, there is evidence that l amino acids are slightly more soluble than a racemic mixture of d and l amino acids, which tend to form crystals. This small solubility difference could have been amplified over time so that the l isomer became dominant in solution.
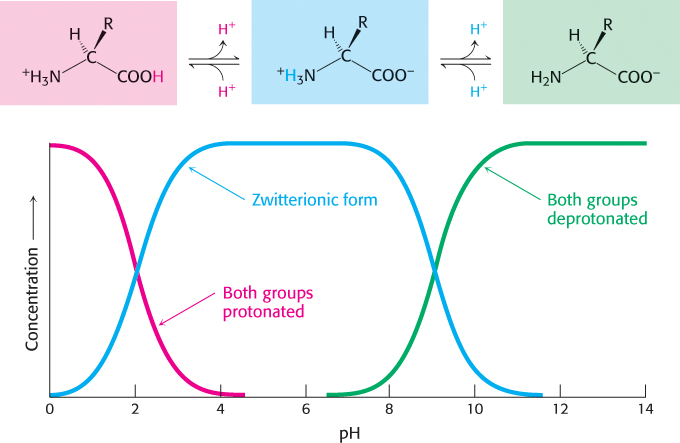
Amino acids in solution at neutral pH exist predominantly as dipolar ions (also called zwitterions). In the dipolar form, the amino group is protonated
30
( NH3+) and the carboxyl group is deprotonated (
NH3+) and the carboxyl group is deprotonated ( COO−). The ionization state of an amino acid varies with pH (Figure 2.6). In acid solution (e.g., pH 1), the amino group is protonated (
COO−). The ionization state of an amino acid varies with pH (Figure 2.6). In acid solution (e.g., pH 1), the amino group is protonated ( NH3+) and the carboxyl group is not dissociated (
NH3+) and the carboxyl group is not dissociated ( COOH). As the pH is raised, the carboxylic acid is the first group to give up a proton, inasmuch as its pKa is near 2. The dipolar form persists until the pH approaches 9, when the protonated amino group loses a proton.
COOH). As the pH is raised, the carboxylic acid is the first group to give up a proton, inasmuch as its pKa is near 2. The dipolar form persists until the pH approaches 9, when the protonated amino group loses a proton.
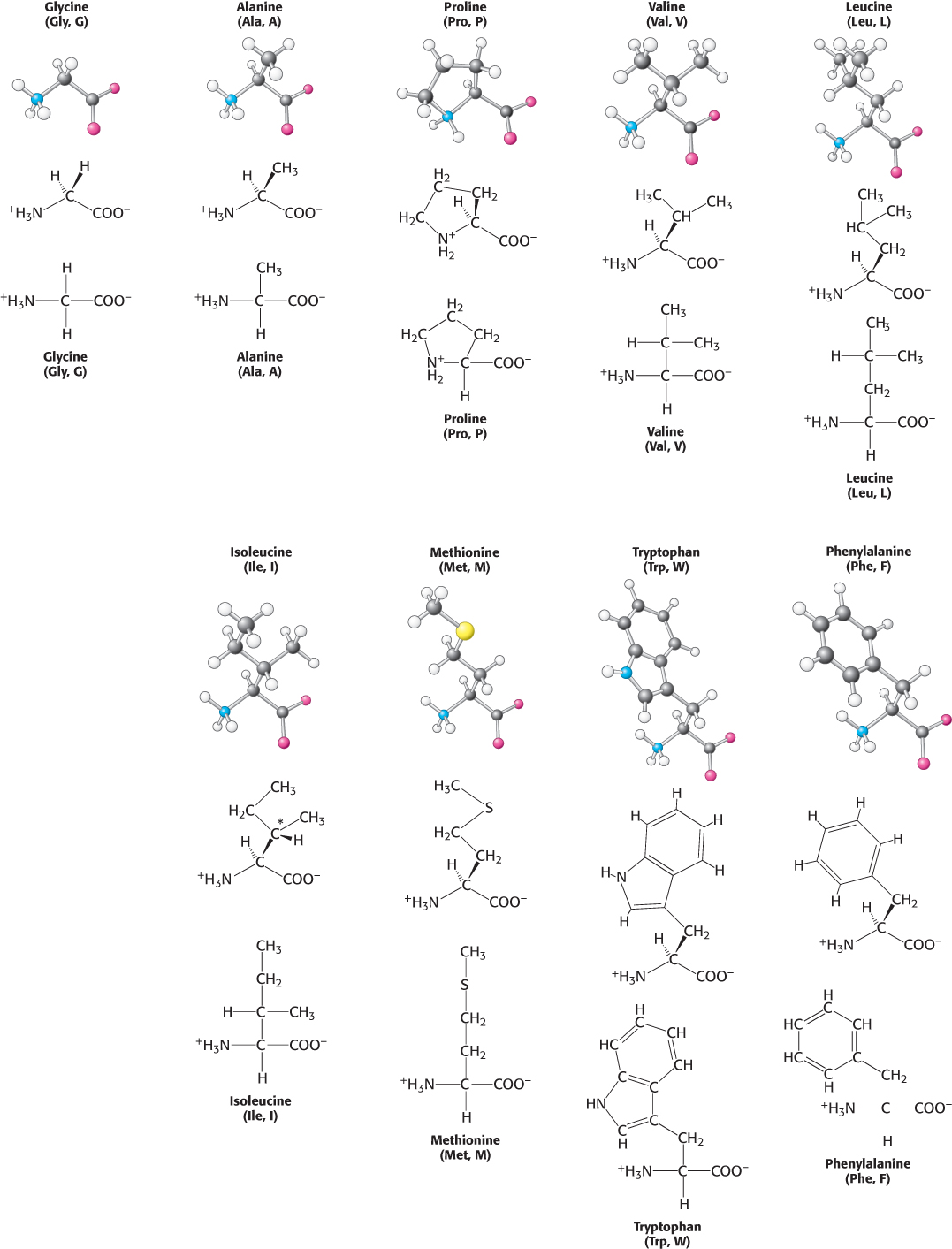
Twenty kinds of side chains varying in size, shape, charge, hydrogen-
Although there are many ways to classify amino acids, we will sort these molecules into four groups, on the basis of the general chemical characteristics of their R groups:
Hydrophobic amino acids with nonpolar R groups
Polar amino acids with neutral R groups but the charge is not evenly distributed
Positively charged amino acids with R groups that have a positive charge at physiological pH
Negatively charged amino acids with R groups that have a negative charge at physiological pH
Hydrophobic amino acids.
The simplest amino acid is glycine, which has a single hydrogen atom as its side chain. With two hydrogen atoms bonded to the α-carbon atom, glycine is unique in being achiral. Alanine, the next simplest amino acid, has a methyl group ( CH3) as its side chain (Figure 2.7).
CH3) as its side chain (Figure 2.7).
31
32
Larger hydrocarbon side chains are found in valine, leucine, and isoleucine. Methionine contains a largely aliphatic side chain that includes a thioether ( S
S ) group. The side chain of isoleucine includes an additional chiral center; only the isomer shown in Figure 2.7 is found in proteins. The larger aliphatic side chains are especially hydrophobic; that is, they tend to cluster together rather than contact water. The three-
) group. The side chain of isoleucine includes an additional chiral center; only the isomer shown in Figure 2.7 is found in proteins. The larger aliphatic side chains are especially hydrophobic; that is, they tend to cluster together rather than contact water. The three-
Two amino acids with relatively simple aromatic side chains are part of the fundamental repertoire. Phenylalanine, as its name indicates, contains a phenyl ring attached in place of one of the hydrogen atoms of alanine. Tryptophan has an indole group joined to a methylene ( CH2
CH2 ) group; the indole group comprises two fused rings containing an NH group. Phenylalanine is purely hydrophobic, whereas tryptophan is less so because of its NH group.
) group; the indole group comprises two fused rings containing an NH group. Phenylalanine is purely hydrophobic, whereas tryptophan is less so because of its NH group.
Polar amino acids.
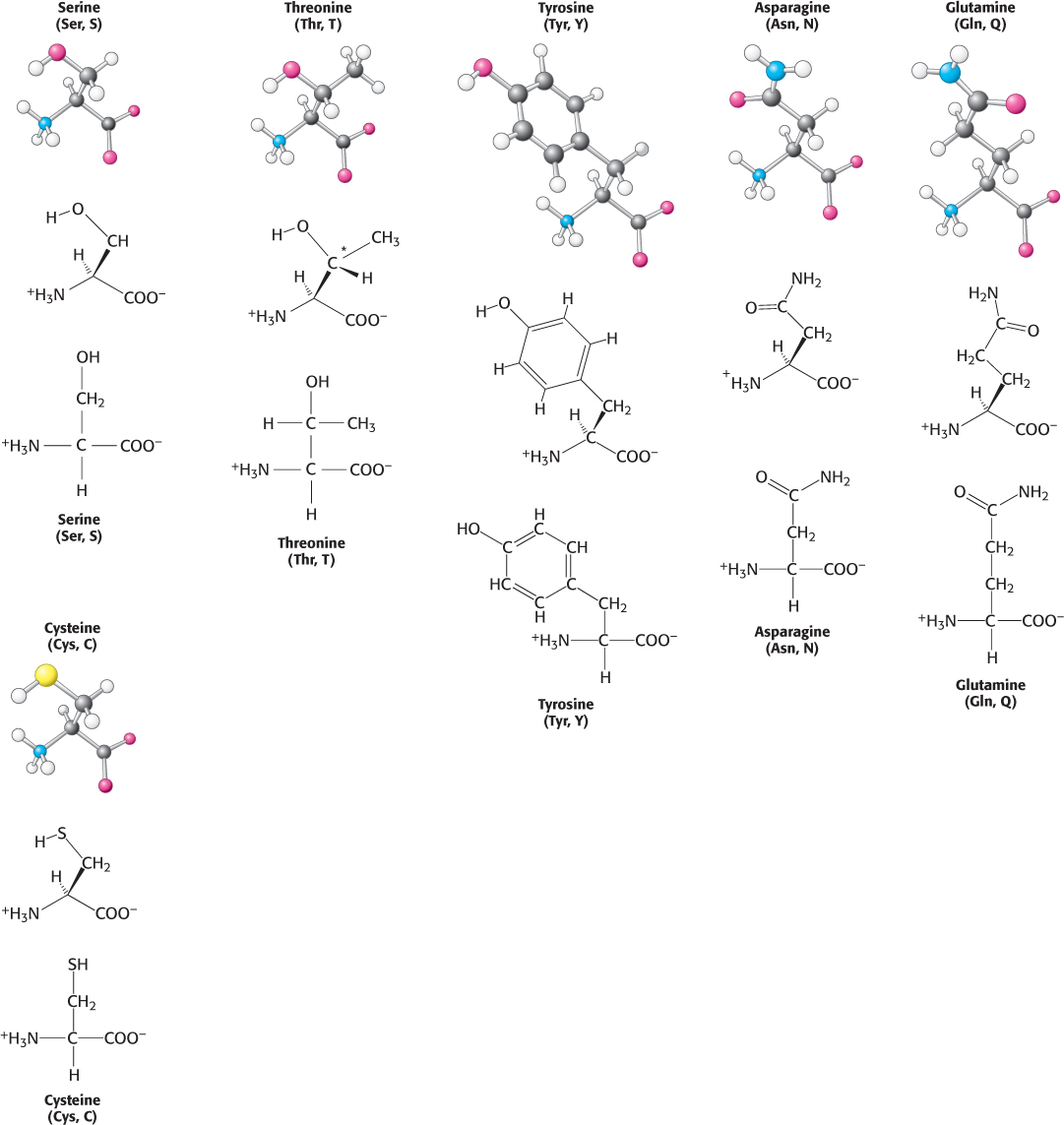
Six amino acids are polar but uncharged. Three amino acids, serine, threonine, and tyrosine, contain hydroxyl groups ( OH) attached to a hydrophobic side chain (Figure 2.8). Serine can be thought of as a version of alanine with a hydroxyl group attached, threonine resembles valine with a hydroxyl group in place of one of valine’s methyl groups, and tyrosine is a version of phenylalanine with the hydroxyl group replacing a hydrogen atom on the aromatic ring. The hydroxyl group makes these amino acids much more hydrophilic (water loving) and reactive than their hydrophobic analogs. Threonine, like isoleucine, contains an additional asymmetric center; again, only one isomer is present in proteins.
OH) attached to a hydrophobic side chain (Figure 2.8). Serine can be thought of as a version of alanine with a hydroxyl group attached, threonine resembles valine with a hydroxyl group in place of one of valine’s methyl groups, and tyrosine is a version of phenylalanine with the hydroxyl group replacing a hydrogen atom on the aromatic ring. The hydroxyl group makes these amino acids much more hydrophilic (water loving) and reactive than their hydrophobic analogs. Threonine, like isoleucine, contains an additional asymmetric center; again, only one isomer is present in proteins.
33
In addition, the set includes asparagine and glutamine, two amino acids that contain a terminal carboxamide. The side chain of glutamine is one methylene group longer than that of asparagine.
Cysteine is structurally similar to serine but contains a sulfhydryl, or thiol ( SH), group in place of the hydroxyl (
SH), group in place of the hydroxyl ( OH) group. The sulfhydryl group is much more reactive. Pairs of sulfhydryl groups may come together to form disulfide bonds, which are particularly important in stabilizing some proteins, as will be discussed shortly.
OH) group. The sulfhydryl group is much more reactive. Pairs of sulfhydryl groups may come together to form disulfide bonds, which are particularly important in stabilizing some proteins, as will be discussed shortly.
Positively charged amino acids.
We turn now to amino acids with complete positive charges that render them highly hydrophilic. Lysine and arginine have long side chains that terminate with groups that are positively charged at neutral pH. Lysine is capped by a primary amino group and arginine by a guanidinium group. Histidine contains an imidazole group, an aromatic ring that also can be positively charged (Figure 2.9).
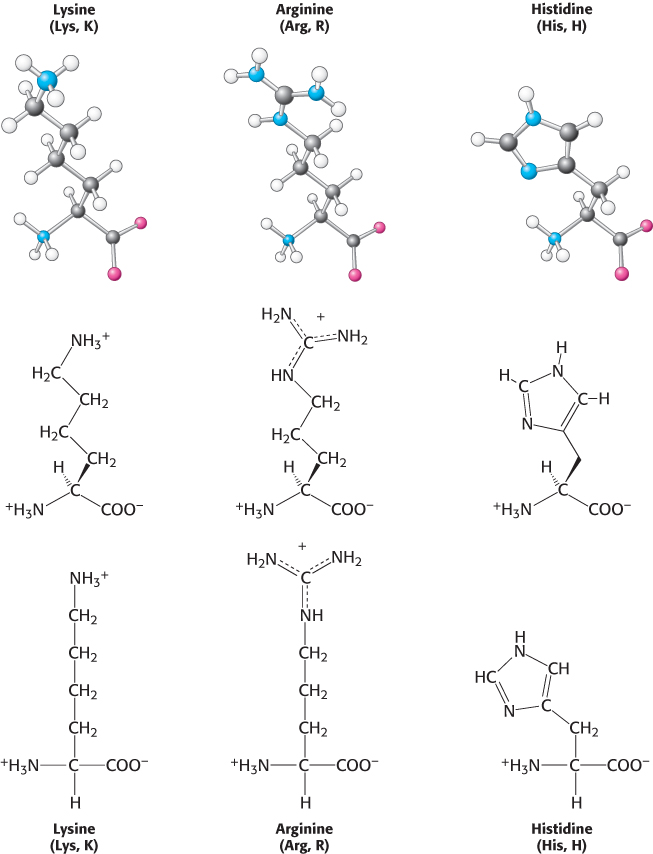

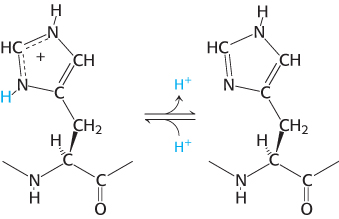
With a pKa value near 6, the imidazole group can be uncharged or positively charged near neutral pH, depending on its local environment (Figure 2.10). Histidine is often found in the active sites of enzymes, where the imidazole ring can bind and release protons in the course of enzymatic reactions.
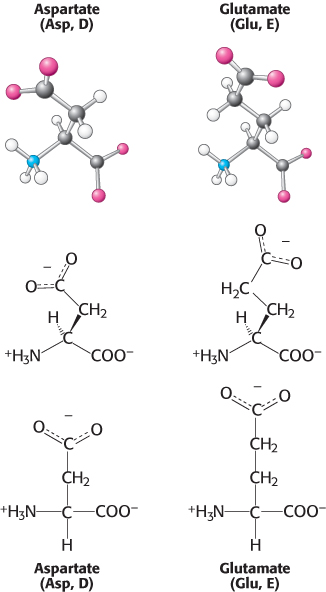
Negatively charged amino acids.
This set of amino acids contains two with acidic side chains: aspartic acid and glutamic acid (Figure 2.11). These amino acids are charged derivatives of asparagine and glutamine (Figure 2.8), with a carboxylic acid in place of a carboxamide. Aspartic acid and glutamic acid are often called aspartate and glutamate to emphasize that, at physiological pH, their side chains usually lack a proton that is present in the acid form and hence are negatively charged. Nonetheless, these side chains can accept protons in some proteins, often with functionally important consequences.
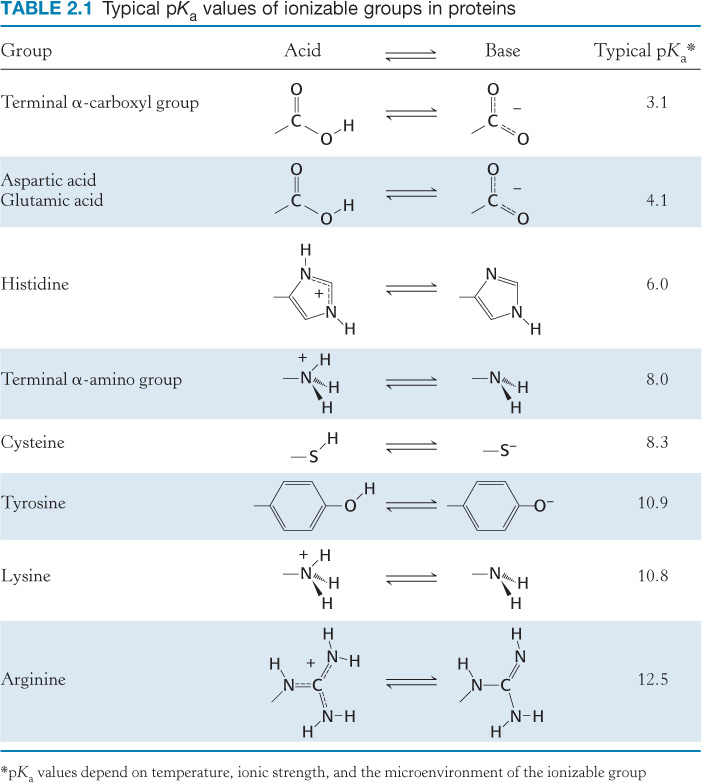
Seven of the 20 amino acids have readily ionizable side chains. These 7 amino acids are able to donate or accept protons to facilitate reactions as well as to form ionic bonds. Table 2.1 gives equilibria and typical pKa values for ionization of the side chains of tyrosine, cysteine, arginine, lysine, histidine, and aspartic and glutamic acids in proteins. Two other groups in proteins—
34
Amino acids are often designated by either a three-
|
Amino acid |
Three- |
One- |
|---|---|---|
|
Alanine |
Ala |
A |
|
Arginine |
Arg |
R |
|
Asparagine |
Asn |
N |
|
Aspartic acid |
Asp |
D |
|
Cysteine |
Cys |
C |
|
Glutamine |
Gln |
Q |
|
Glutamic acid |
Glu |
E |
|
Glycine |
Gly |
G |
|
Histidine |
His |
H |
|
Isoleucine |
Ile |
I |
|
Leucine |
Leu |
L |
|
Lysine |
Lys |
K |
|
Methionine |
Met |
M |
|
Phenylalanine |
Phe |
F |
|
Proline |
Pro |
P |
|
Serine |
Ser |
S |
|
Threonine |
Thr |
T |
|
Tryptophan |
Trp |
W |
|
Tyrosine |
Tyr |
Y |
|
Valine |
Val |
V |
|
Asparagine or aspartic acid |
Asx |
B |
|
Glutamine or glutamic acid |
Glx |
Z |
35
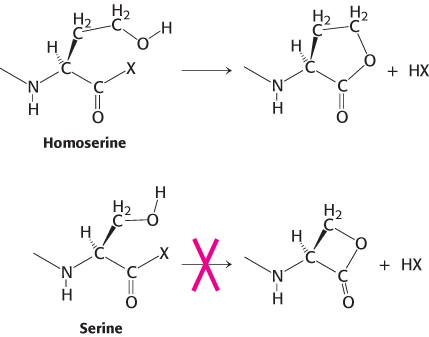
 How did this particular set of amino acids become the building blocks of proteins? First, as a set, they are diverse: their structural and chemical properties span a wide range, endowing proteins with the versatility to assume many functional roles. Second, many of these amino acids were probably available from prebiotic reactions; that is, from reactions that took place before the origin of life. Finally, other possible amino acids may have simply been too reactive. For example, amino acids such as homoserine and homocysteine tend to form five-
How did this particular set of amino acids become the building blocks of proteins? First, as a set, they are diverse: their structural and chemical properties span a wide range, endowing proteins with the versatility to assume many functional roles. Second, many of these amino acids were probably available from prebiotic reactions; that is, from reactions that took place before the origin of life. Finally, other possible amino acids may have simply been too reactive. For example, amino acids such as homoserine and homocysteine tend to form five-