Chapter 18
Chapter 18
1. In fermentations, organic compounds are both the donors and the acceptors of electrons. In respiration, the electron donor is usually an organic compound, whereas the electron acceptor is an inorganic molecule, such as oxygen.
2. Biochemists use 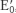 , the value at pH 7, whereas chemists use E0, the value in 1 M H+. The prime denotes that pH 7 is the standard state.
, the value at pH 7, whereas chemists use E0, the value in 1 M H+. The prime denotes that pH 7 is the standard state.
3. The reduction potential of FADH2 is less than that of NADH (Table 18.1). Consequently, when those electrons are passed along to oxygen, less energy is released. The consequence of the difference is that electron flow from FADH2 to O2 pumps fewer protons than do the electrons from NADH.
4. The ΔG°′ for the reduction of oxygen by FADH2 is −200 kJ mol−1 (−48 kcal mol−1).
5. ΔG°′ is + 67 kJ mol−1 (+16.1 kcal mol−1) for oxidation by NAD+ and −3.8 kJ mol−1 (−0.92 kcal mol−1) for oxidation by FAD. The oxidation of succinate by NAD+ is not thermodynamically feasible.
6. An oxidizing agent, or oxidant, accepts electrons in oxidation–
7. Pyruvate accepts electrons and is thus the oxidant. NADH gives up electrons and is the reductant.
8. 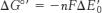
9. The 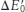 value of iron can be altered by changing the environment of the ion.
value of iron can be altered by changing the environment of the ion.
10. c, e, b, a, d.
11. (a) 4; (b) 5; (c) 2; (d) 10; (e) 3; (f) 8; (g) 9; (h) 7; (i) 1; (j) 6.
12. (a) 4; (b) 3; (c) 1; (d) 5; (e) 2.
13. The 10 isoprene units render coenzyme Q soluble in the hydrophobic environment of the inner mitochondrial membrane. The two oxygen atoms can reversibly bind two electrons and two protons as the molecule transitions between the quinone form and quinol form.
14. Rotenone: NADH, NADH-
15. Complex I would be reduced, whereas Complexes II, III, and IV would be oxidized. The citric acid cycle would halt because it has no way to oxidize NADH.
16. The respirasome is another example of the use of supramolecular complexes in biochemistry. Having the three complexes that are proton pumps associated with one another will enhance the efficiency of electron flow from complex to complex, which in turn will cause more-
17. Succinate dehydrogenase is a component of Complex II.
18. Hydroxyl radical (OH ⋅), hydrogen peroxide (H2O2), superoxide ion (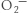 ), and peroxide (
), and peroxide (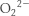 ). These small molecules react with a host of macromolecules—
). These small molecules react with a host of macromolecules—
19. The ATP is recycled by ATP-
20. (a) 12.5; (b) 14; (c) 32; (d) 13.5; (e) 30; (f ) 16.
21. (a) It blocks electron transport and proton pumping at Complex IV. (b) It blocks electron transport and ATP synthesis by inhibiting the exchange of ATP and ADP across the inner mitochondrial membrane. (c) It blocks electron transport and proton pumping at Complex I. (d) It blocks ATP synthesis without inhibiting electron transport by dissipating the proton gradient. (e) It blocks electron transport and proton pumping at Complex IV. (f ) It blocks electron transport and proton pumping at Complex III.
22. If the proton gradient is not dissipated by the influx of protons into a mitochondrion with the generation of ATP, eventually the outside of the mitochondrion develops such a large positive charge that the electron-
23. The subunits are jostled by background thermal energy (Brownian motion). The proton gradient makes clockwise rotation more likely because that direction results in protons flowing down their concentration gradient.
24. Dicyclohexylcarbodiimide reacts readily with carboxyl groups. Hence, the most likely targets are aspartate and glutamate side chains. In fact, Asp 61 of subunit c of E. coli F0 is specifically modified by this reagent. The conversion of Asp 61 into asparagine by site-
25. In the presence of poorly functioning mitochondria, the only means of generating ATP is by anaerobic glycolysis, which will lead to an accumulation of lactic acid in blood.
26. If ADP cannot get into mitochondria, the electron-
A24
27. (a) No effect; mitochondria cannot metabolize glucose.
(b) No effect; no fuel is present to power the synthesis of ATP.
(c) The [O2] falls because citrate is a fuel and ATP can be formed from ADP and Pi.
(d) Oxygen consumption stops because oligomycin inhibits ATP synthesis, which is coupled to the activity of the electron-
(e) No effect, for the reasons given in part d.
(f) [O2] falls rapidly because the system is uncoupled and does not require ATP synthesis to lower the proton-
(g) [O2] falls, though at a lower rate. Rotenone inhibits Complex I, but the presence of succinate will enable electrons to enter at Complex II.
(h) Oxygen consumption ceases because Complex IV is inhibited and the entire chain backs up.
28. (a) The P : O ratio is equal to the product of (H+/2e−) and (P/H+). Note that the P : O ratio is identical with the P : 2 e− ratio.
(b) 2.5 and 1.5, respectively.
29. Cyanide can be lethal because it binds to the ferric form of cytochrome oxidase and thereby inhibits oxidative phosphorylation. Nitrite converts ferrohemoglobin into ferrihemoglobin, which also binds cyanide. Thus, ferrihemoglobin competes with cytochrome c oxidase for cyanide. This competition is therapeutically effective because the amount of ferrihemoglobin that can be formed without impairing oxygen transport is much greater than the amount of cytochrome c oxidase.
30. Such a defect (called Luft syndrome) was found in a 38-
31. Triose phosphate isomerase converts dihydroxyacetone phosphate (a potential dead end) into glyceraldehyde 3-
32. This inhibitor (like antimycin A) blocks the reduction of cytochrome c1 by QH2, the crossover point.
33. If oxidative phosphorylation were uncoupled, no ATP could be produced. In a futile attempt to generate ATP, much fuel would be consumed. The danger lies in the dose. Too much uncoupling would lead to tissue damage in highly aerobic organs such as the brain and heart, which would have severe consequences for the organism as a whole. The energy that is normally transformed into ATP would be released as heat. To maintain body temperature, sweating might increase, although the very process of sweating itself depends on ATP.
34. If ATP and ADP cannot exchange between the matrix and the mitochondria, ATP synthase will cease to function because its substrate ADP is absent. The proton gradient will eventually become so large that the energy released by the electron-
35. Add the inhibitor with and without an uncoupler, and monitor the rate of O2 consumption. If the O2 consumption increases again in the presence of inhibitor and uncoupler, the inhibitor must be inhibiting ATP synthase. If the uncoupler has no effect on the inhibition, the inhibitor is inhibiting the electron-
36. Presumably, because the muscle has greater energy needs, especially during exercise, it will require more ATP. This requirement means that more sites of oxidative phosphorylation are called for, and these sites can be provided by an increase in the amount of cristae.
37. The arginine residue, with its positive charge, will facilitate proton release from aspartic acid by stabilizing the negatively charged aspartate.
38. 4; 4.7
39. The ATP synthase would pump protons at the expense of ATP hydrolysis, thus maintaining the proton-
40. It suggests that malfunctioning mitochondria may play a role in the development of Parkinson disease. Specifically, it implicates Complex I.
41. The extra negative charge on ATP relative to that on ADP accounts for ATP’s more-
42. When all of the available ADP has been converted into ATP, ATP synthase can no longer function. The proton gradient becomes large enough that the energy of the electron-
43. The effect on the proton gradient is the same in each case.
44. ATP export from the matrix. Phosphate import into the matrix.
45. Recall from the discussion of enzyme-
46. The cytoplasmic kinases thereby obtain preferential access to the exported ATP.
47. The organic acids in the blood are indications that the mice are deriving a large part of their energy needs through anaerobic glycolysis. Lactate is the end product of anaerobic glycolysis. Alanine is an aminated transport form of pyruvate, which is formed from lactate. Alanine formation plays a role in succinate formation, which is caused by the reduced state of the mitochondria.

The electron-
Indeed, these mice display evidence of such oxidative damage.
48. (a) Vitamins C and E.
(b) Exercise induces superoxide dismutase, which converts ROS in hydrogen peroxide and oxygen.
A25
(c) The answer to this question is not fully established. Two possibilities are (1) the suppression of ROS by vitamins prevents the expression of more superoxide dismutase and (2) some ROS may be signal molecules required to stimulate insulin-
49. (a) DNP is an uncoupler that prevents the use of the proton-
(b) Because glycolysis is now inhibited, no lactic acid will be produced and the rate of extracellular acidification will fall. Because DNP is still present, oxygen consumption will still occur at a high rate.
(c) A key step in glycolysis is the isomerization of glucose 6-
(d) Rotenone inhibits electron flow through Complex I, the electron transport chain is inhibited and oxygen consumption ceases.
50. (a) Succinate is oxidized by Complex II, and the electrons are used to establish a proton-
(b) The ability to synthesize ATP is greatly reduced.
(c) The goal was to measure ATP hydrolysis. If succinate had been added in the presence of ATP, no reaction would have taken place, because of respiratory control.
(d) The mutation has little effect on the ability of the enzyme to catalyze the hydrolysis of ATP.
(e) They suggest two things: (1) the mutation did not affect the catalytic site on the enzyme, because ATP synthase is still capable of catalyzing the reverse reaction, and (2) the mutation did not affect the amount of enzyme present, given that the controls and patients had similar amounts of activity.
51. The absolute configuration of thiophosphate indicates that inversion at phosphorus has taken place in the reaction catalyzed by ATP synthase. This result is consistent with an inline phosphoryl-