Chapter 22
Chapter 22
1. (a) 5; (b) 11; (c) 1; (d) 10; (e) 2; (f) 6; (g) 9; (h) 3; (i) 4; (j) 7; (k) 8.
2.
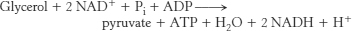
Glycerol kinase and glycerol phosphate dehydrogenase
3. The ready reversibility is due to the high-
4. To return the AMP to a form that can be phosphorylated by oxidative phosphorylation or substrate-
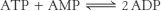
5. b, c, a, g, h, d, e, f.
6. The citric acid cycle. The reactions that take succinate to oxaloacetate, or the reverse, are similar to those of fatty acid metabolism (Section 17.2).
7. The next-
8. Palmitic acid yields 106 molecules of ATP. Palmitoleic acid has a double bond between carbons C-
9.
|
Activation fee to form the acyl CoA |
−2 ATP |
|
Seven rounds of yield: |
|
|
7 acetyl CoA at 10 ATP/acetyl CoA |
+ 70 ATP |
|
7 NADH at 2.5 ATP/NADH |
+ 17.5 ATP |
|
7 FADH2 at 1.5 ATP/FADH2 |
+ 10.5 ATP |
|
Propionyl CoA, which requires an ATP to be converted into succinyl CoA |
− 1 ATP |
|
Succinyl CoA → succinate |
+ 1 ATP |
|
Succinate → fumarate → FADH2 FADH2 at 1.5 ATP/FADH2 |
+ 1.5 ATP |
|
Fumarate → malate |
|
|
Malate → oxaloacetate + NADH NADH at 2.5 ATP/NADH |
+ 2.5 ATP |
|
Total |
120 ATP |
A30
10. You might hate yourself in the morning, but at least you won’t have to worry about energy. To form stearoyl CoA requires the equivalent of 2 molecules of ATP.
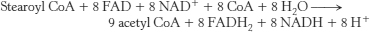
|
9 acetyl CoA at 10 ATP/acetyl CoA |
+ 90 ATP |
|
8 NADH at 2.5 ATP/NADH |
+ 20 ATP |
|
8 FADH2 at 1.5 ATP/FADH2 |
+ 12 ATP |
|
Activation fee |
−2.0 |
|
Total |
122 ATP |
11. Keep in mind that, in the citric acid cycle, 1 molecule of FADH2 yields 1.5 ATP, 1 molecule of NADH yields 2.5 ATP, and 1 molecule of acetyl CoA yields 10 ATP. Two molecules of ATP are produced when glucose is degraded to 2 molecules of pyruvate. Two molecules of NADH also are produced, but the electrons are transferred to FADH2 to enter the electron transport chain. Each molecule of FADH2 can generate 1.5 ATP. Each molecule of pyruvate will produce 1 molecule of NADH. Each molecule of acetyl CoA generates 3 molecules of NADH, 1 molecule of FADH2, and 1 molecule of ATP. So, we have a total of 10 ATP per acetyl CoA, or 20 for the 2 molecules of acetyl CoA. The total for glucose is 30 ATP. Now, what about hexanoic acid? Caprioic acid is activated to caprioic CoA at the expense of 2 ATP, and so we are 2 ATP in the hole. The first cycle of β oxidation generates 1 FADH2, 1 NADH, and 1 acetyl CoA. After the acetyl CoA has been run through the citric acid cycle, this step will have generated a total of 14 ATP. The second cycle of β oxidation generates 1 FADH2 and 1 NADH but 2 acetyl CoA. After the acetyl CoA has been run through the citric acid cycle, this step will have generated a total of 24 ATP. The total is 36 ATP. Thus, the foul-
12. Stearate + ATP + 13.5 H2O + 8 FAD + 8 NAD+ → 4.5 acetoacetate + 14.5 H+ + 8 FADH2 + 8 NADH + AMP + 2 Pi.
13. Palmitate is activated and then processed by β oxidation according to the following reactions.
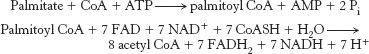
The eight molecules of acetyl CoA combine to form four molecules of acetoacetate for release into the blood, and so they do not contribute to the energy yield in the liver. However, the FADH2 and NADH generated in the preparation of acetyl CoA can be processed by oxidative phosphorylation to yield ATP.
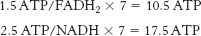
The equivalent of 2 ATP were used to form palmitoyl CoA. Thus, 26 ATP were generated for use by the liver.
14. NADH produced with the oxidation to acetoacetate = 2.5 ATP. Acetoacetate is converted into acetoacetyl CoA. Two molecules of acetyl CoA result from the hydrolysis of acetoacetyl CoA, each worth 10 ATP when processed by the citric acid cycle. Total ATP yield is 22.5.
15. Because a molecule of succinyl CoA is used to form acetoacetyl CoA. Succinyl CoA could be used to generate one molecule of ATP, and so someone could argue that the yield is 21.5.
16. For fats to be combusted, not only must they be converted into acetyl CoA, but the acetyl CoA must be processed by the citric acid cycle. In order for acetyl CoA to enter the citric acid cycle, there must be a supply of oxaloacetate. Oxaloacetate can be formed by the metabolism of glucose to pyruvate and the subsequent carboxylation of pyruvate to form oxaloacetate.
17. (a)
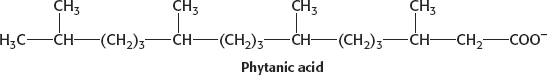
The problem with phytanic acid is that, as it undergoes β oxidation, we encounter the dreaded pentavalent carbon atom. Because the pentavalent carbon atom doesn’t exist, β oxidation cannot take place and phytanic acid accumulates.
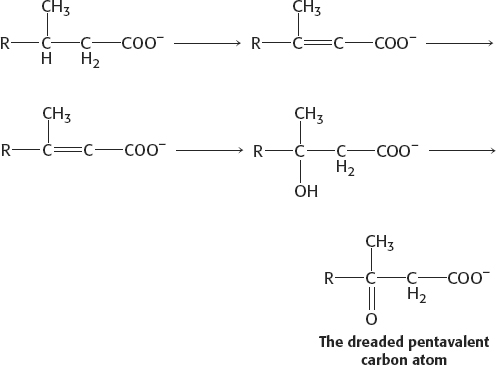
(b) Removing methyl groups, though theoretically possible, would be time consuming and lacking in elegance. What would we do with the methyl groups? Our livers solve the problem by inventing α oxidation.
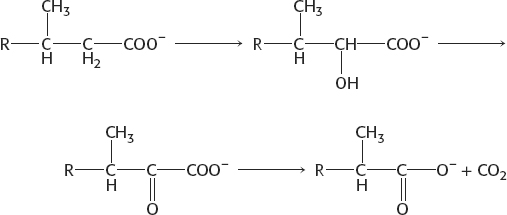
One round of α oxidation rather than β oxidation converts phytanic acid into a β-oxidation substrate.
18. The first oxidation removes two tritium atoms. The hydration adds nonradioactive H and OH. The second oxidation removes another tritium atom from the β-carbon atom. Thiolysis removes an acetyl CoA with only one tritium atom; so the tritium-
A31
19. In the absence of insulin, lipid mobilization will take place to an extent that it overwhelms the ability of the liver to convert the lipids into ketone bodies.
20. (a) 10; (b) 1; (c) 5; (d) 8; (e) 3; (f) 9; (g) 6; (h) 7; (i) 4; (j) 2.
21. (a) Oxidation in mitochondria; synthesis in the cytoplasm.
(b) Coenzyme A in oxidation; acyl carrier protein for synthesis.
(c) FAD and NAD+ in oxidation; NADPH for synthesis.
(d) The l isomer of 3-
(e) From carboxyl to methyl in oxidation; from methyl to carboxyl in synthesis.
(f) The enzymes of fatty acid synthesis, but not those of oxidation, are organized in a multienzyme complex.
22. Bicarbonate is required for the synthesis of malonyl CoA from acetyl CoA by acetyl CoA carboxylase.
23. 
24. We will need six acetyl CoA units. One acetyl CoA unit will be used directly to become the two carbon atoms farthest from the acid end. The other five units must be converted into malonyl CoA. The synthesis of each malonyl CoA molecule costs a molecule of ATP; so 5 molecules of ATP will be required. Each round of elongation requires 2 molecules of NADPH, 1 molecule to reduce the keto group to an alcohol and 1 molecule to reduce the double bond. As a result, 10 molecules of NADPH will be required. Therefore, 5 molecules of ATP and 10 molecules of NADPH are required to synthesize lauric acid.
25. e, b, d, a, c.
26. Such a mutation would inhibit fatty acid synthesis because the enzyme cleaves cytoplasmic citrate to yield acetyl CoA for fatty acid synthesis.
27. (a) False. Biotin is required for acetyl CoA carboxylase activity.
(b) True.
(c) False. ATP is required to synthesize malonyl CoA.
(d) True.
(e) True.
(f) False. Fatty acid synthase is a dimer.
(g) True.
(h) False. Acetyl CoA carboxylase is stimulated by citrate, which is cleaved to yield its substrate acetyl CoA.
28. Fatty acids with odd numbers of carbon atoms are synthesized starting with propionyl ACP (instead of acetyl ACP), which is formed from propionyl CoA by acetyl transacetylase.
29. All of the labeled carbon atoms will be retained. Because we need 8 acetyl CoA molecules and only 1 carbon atom is labeled in the acetyl group, we will have 8 labeled carbon atoms. The only acetyl CoA used directly will retain 3 tritium atoms. The 7 acetyl CoA molecules used to make malonyl CoA will lose 1 tritium atom on addition of the CO2 and another one at the dehydration step. Each of the 7 malonyl CoA molecules will retain 1 tritium atom. Therefore, the total retained tritium is 10 atoms. The ratio of tritium to carbon is 1.25.
30. With a diet rich in raw eggs, avidin will inhibit fatty acid synthesis by reducing the amount of biotin required by acetyl CoA carboxylase. Cooking the eggs will denature avidin, and so it will no longer bind biotin.
31. The only acetyl CoA used directly, not in the form of malonyl CoA, provides the two carbon atoms at the ω end of the fatty acid chain. Because palmitic acid is a C16 fatty acid, acetyl CoA will have provided carbons 15 and 16.
32. HCO3− is attached to acetyl CoA to form malonyl CoA. When malonyl CoA condenses with acetyl CoA to form the four-
33. Phosphofructokinase controls the flux down the glycolytic pathway. Glycolysis functions to generate ATP or building blocks for biosynthesis, depending on the tissue. The presence of citrate in the cytoplasm indicates that those needs are met, and there is no need to metabolize glucose.
34. C-
35. The mutant enzyme will be persistently active because it cannot be inhibited by phosphorylation. Fatty acid synthesis will be abnormally active. Such a mutation might lead to obesity.
36. (a) Palmitoleate; (b) linoleate; (c) linoleate; (d) oleate; (e) oleate; (f) linolenate.
37. Decarboxylation drives the condensation of malonyl ACP and acetyl ACP. In contrast, the condensation of two molecules of acetyl ACP is energetically unfavorable. In gluconeogenesis, decarboxylation drives the formation of phosphoenolpyruvate from oxaloacetate.
38. Fat mobilization in adipocytes is activated by phosphorylation. Hence, overproduction of the cAMP-
39. Carnitine translocase deficiency and glucose 6-
40. In the fifth round of β oxidation, cis-Δ2-enoyl CoA is formed. Dehydration by the classic hydratase yields d-3-
41. An advantage of this arrangement is that the synthetic activity of different enzymes is coordinated. In addition, intermediates can be efficiently handed from one active site to another without leaving the assembly. Furthermore, a complex of covalently joined enzymes is more stable than one formed by noncovalent attractions. Each of the component enzymes is recognizably homologous to its bacterial counterpart.
42. The probability of synthesizing an error-
43. The absence of ketone bodies is due to the fact that the liver, the source of ketone bodies in the blood, cannot oxidize fatty acids to produce acetyl CoA. Moreover, because of the impaired fatty acid oxidation, the liver becomes more dependent on glucose as an energy source. This dependency results in a decrease in gluconeogenesis and a drop in blood-
44. Peroxisomes enhance the degradation of very long chain fatty acids. Consequently, increasing the activity of peroxisomes could help to lower levels of blood triglycerides. In fact, clofibrate is rarely used because of serious side effects.
45. Citrate works by facilitating, in cooperation with the protein MIG12, the formation of active filaments from inactive monomers. In essence, it increases the number of active sites available, or the concentration of enzyme. Consequently, its effect is visible as an increase in the value of Vmax. Allosteric enzymes that alter their Vmax values in response to regulators are sometimes called V-
A32
46. The thiolate anion of CoA attacks the 3-
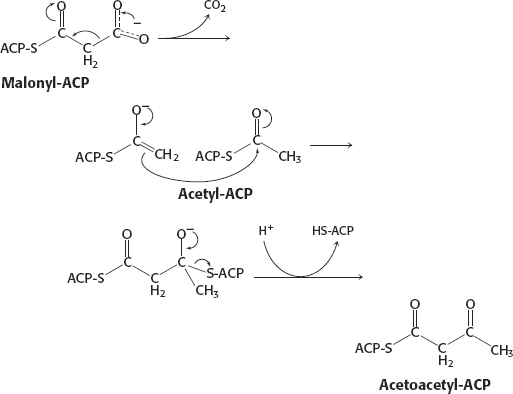
47.
48. (a) Fats burn in the flame of carbohydrates. Without carbohydrates, there would be no anapleurotic reactions to replenish the components of the citric acid cycle. With a diet of fats only, the acetyl CoA from fatty acid degradation would build up.
(b) Acetone from ketone bodies.
(c) Yes. Odd-
49. A labeled fat can enter the citric acid cycle as acetyl CoA and yield labeled oxaloacetate, but only after two carbon atoms have been lost as CO2. Consequently, even though oxaloacetate may be labeled, there can be no net synthesis of oxaloacetate and hence no net synthesis of glucose or glycogen.
50. (a) Glucose is the primary fuel used by the brain. Lack of pyruvate dehydrogenase would prevent complete oxidation of glucose-
51. I-
52. (a) The Vmax is decreased and the Km is increased. Vmax (wild type) = 13 nmol minute−1 mg−1; Km (wild type) = 45 μM; Vmax (mutant) = 8.3 nmol minute−1 mg−1; Km (muttxt_margin_0) = 74 μM.
(b) Both the Vmax and the Km are decreased. Vmax (wild type) = 41 nmol minute−1 mg−1; Km (wild type) = 104 μM; Vmax (mutant) = 23 nmol minute−1 mg−1; Km (mutant) = 69txt_margin_0956;M.
(c) The wild type is significantly more sensitive to malonyl CoA.
(d) With respect to carnitine, the mutant displays approximately 65% of the activity of the wild type; with respect to palmitoyl CoA, approximately 50% activity. On the other hand, 10 μM of malonyl CoA inhibits approximately 80% of the wild type but has essentially no effect on the mutant enzyme.
(e) The glutamate appears to play a more prominent role in regulation by malonyl CoA than in catalysis.
53. (a) Leucine is changed to alanine.
(b) No change relative to control.
(c) Tyrosine to alanine.
(d) Greatly reduced lipase binding compared to the control.
(e) Lipase activity was diminished.
(f) This suggests that they are separate. The L16A mutation allows binding of the lipase but apparently does not allow activation of the lipase.
(g) This mutation decreased both binding and activity, suggesting that the tyrosine is involved in both aspects of colipase activity.
54. (a) Soraphen A inhibits fatty acid synthesis in a dose-
(b) Fatty acid oxidation is increased in the presence of soraphen A.
(c) Recall that acetyl CoA carboxylase 2 synthesizes malonyl CoA to inhibit the transport of fatty acids into the mitochondria, thereby preventing fatty acid oxidation. Soraphen A apparently inhibits both forms of the carboxylase.
(d) Phospholipid synthesis was inhibited in a dose-
(e) Phospholipids are required for membrane synthesis.
(f) Soraphen A inhibits cell proliferation, especially at higher concentrations.