12.3Phospholipids and Glycolipids Readily Form Bimolecular Sheets in Aqueous Media
Phospholipids and Glycolipids Readily Form Bimolecular Sheets in Aqueous Media
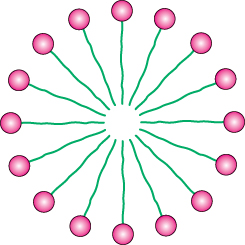
What properties enable phospholipids to form membranes? Membrane formation is a consequence of the amphipathic nature of the molecules.Their polar head groups favor contact with water, whereas their hydrocarbon tails interact with one another in preference to water. How can molecules with these preferences arrange themselves in aqueous solutions? One way is to form a globular structure called a micelle. The polar head groups form the outside surface of the micelle, which is surrounded by water, and the hydrocarbon tails are sequestered inside, interacting with one another (Figure 12.9).
Alternatively, the strongly opposed preferences of the hydrophilic and hydrophobic moieties of membrane lipids can be satisfied by forming a lipid bilayer, composed of two lipid sheets (Figure 12.10). A lipid bilayer is also called a bimolecular sheet. The hydrophobic tails of each individual sheet interact with one another, forming a hydrophobic interior that acts as a permeability barrier. The hydrophilic head groups interact with the aqueous medium on each side of the bilayer. The two opposing sheets are called leaflets.
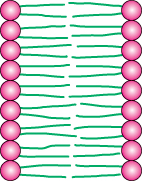
The favored structure for most phospholipids and glycolipids in aqueous media is a bimolecular sheet rather than a micelle. The reason is that the two fatty acid chains of a phospholipid or a glycolipid are too bulky to fit into the interior of a micelle. In contrast, salts of fatty acids (such as sodium palmitate, a constituent of soap) readily form micelles because they contain only one chain. The formation of bilayers instead of micelles by phospholipids is of critical biological importance. A micelle is a limited structure, usually less than 200 Å (20 nm) in diameter. In contrast, a bimolecular sheet can extend to macroscopic dimensions, as much as a millimeter (107 Å, or 106 nm) or more. Phospholipids and related molecules are important membrane constituents because they readily form extensive bimolecular sheets.
Lipid bilayers form spontaneously by a self-
Lipid vesicles can be formed from phospholipids
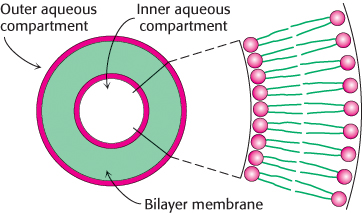
The propensity of phospholipids to form membranes has been used to create an important experimental and clinical tool. Lipid vesicles, or liposomes, are aqueous compartments enclosed by a lipid bilayer (Figure 12.11). These structures can be used to study membrane permeability or to deliver chemicals to cells. Liposomes are formed by suspending a suitable lipid, such as phosphatidylcholine, in an aqueous medium, and then sonicating (i.e., agitating by high-
349
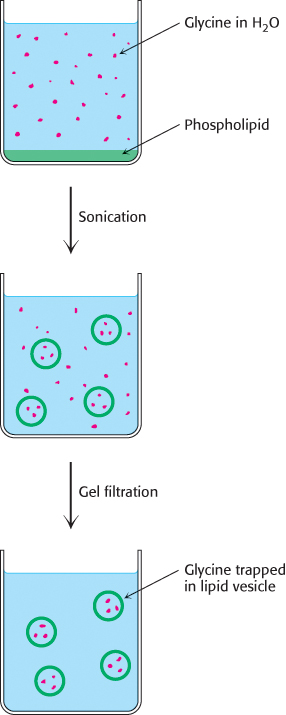
Ions or molecules can be trapped in the aqueous compartments of lipid vesicles by forming the vesicles in the presence of these substances (Figure 12.12). For example, 500-
 Therapeutic applications for liposomes are currently under active investigation. For example, liposomes containing drugs or DNA can be injected into patients. These liposomes fuse with the plasma membrane of many kinds of cells, introducing into the cells the molecules that they contain. Drug delivery with liposomes often lessens its toxicity. Less of the drug is distributed to normal tissues because long-
Therapeutic applications for liposomes are currently under active investigation. For example, liposomes containing drugs or DNA can be injected into patients. These liposomes fuse with the plasma membrane of many kinds of cells, introducing into the cells the molecules that they contain. Drug delivery with liposomes often lessens its toxicity. Less of the drug is distributed to normal tissues because long-
Another well-
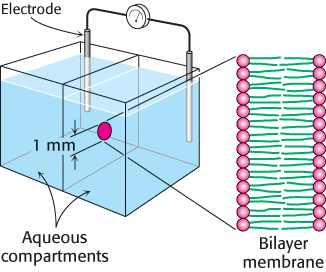
Lipid bilayers are highly impermeable to ions and most polar molecules
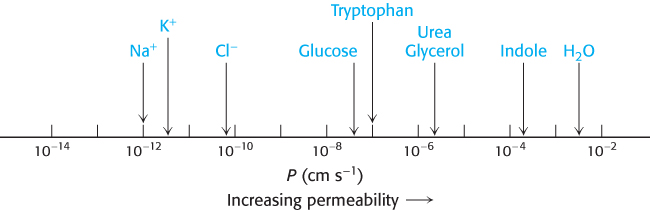
Permeability studies of lipid vesicles and electrical-
350