19.2
Light Absorption by Chlorophyll Induces Electron Transfer
The trapping of light energy is the key to photosynthesis. The first event is the absorption of light by a photoreceptor molecule. The principal photoreceptor in the chloroplasts of most green plants is the pigment molecule chlorophyll a, a substituted tetrapyrrole (Figure 19.5). The four nitrogen atoms of the pyrroles are coordinated to a magnesium ion. Unlike a porphyrin such as heme, chlorophyll has a reduced pyrrole ring and an additional 5-carbon ring fused to one of the pyrrole rings. Another distinctive feature of chlorophyll is the presence of phytol, a highly hydrophobic 20-carbon alcohol, esterified to an acid side chain.
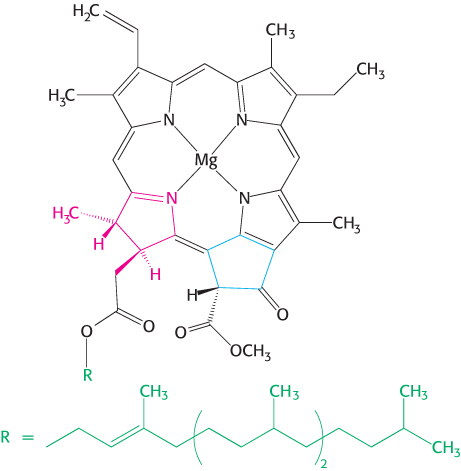
FIGURE 19.5Chlorophyll. Like heme, chlorophyll a is a cyclic tetrapyrrole. One of the pyrrole rings (shown in red) is reduced, and an additional five-carbon ring (shown in blue) is fused to another pyrrole ring. A phytol chain (shown in green) is connected by an ester linkage. Magnesium ion binds at the center of the structure.
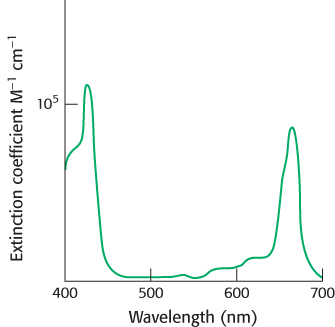
FIGURE 19.6Light absorption by chlorophyll a. Chlorophyll a absorbs visible light efficiently, as judged by the extinction coefficient near 105 M−1 cm−1.
Chlorophylls are very effective photoreceptors because they contain networks of conjugated double bonds—alternating single and double bonds. Such compounds are called conjugated polyenes. In polyenes, the electrons are not localized to a particular atomic nucleus. Upon the absorption of light energy, the electron transitions from a low energy molecular orbital to a higher energy orbital. Chlorophylls have very strong absorption bands in the visible region of the spectrum, where the solar output reaching Earth is maximal (Figure 19.6). Chlorophyll a’s peak molar extinction coefficient (ϵ), a measure of a compound’s ability to absorb light, is greater than 105 M−1 cm−1, among the highest observed for organic compounds.
What happens when light is absorbed by a pigment molecule? The energy from the light excites an electron from its ground energy level to an excited energy level (Figure 19.7). This high-energy electron can have one of two fates. For most compounds that absorb light, the electron simply returns to the ground state and the absorbed energy is converted into heat. However, if a suitable electron acceptor is nearby, as is the case for chlorophyll in photosynthetic systems, the excited electron can move from the initial molecule to the acceptor (Figure 19.8). A positive charge forms on the initial molecule, owing to the loss of an electron, and a negative charge forms on the acceptor, owing to the gain of an electron. Hence, this process is referred to as photoinduced charge separation.
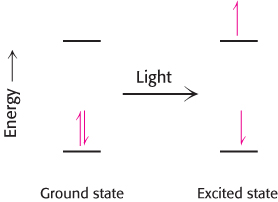
FIGURE 19.7Light absorption. The absorption of light leads to the excitation of an electron from its ground state to a higher energy level.
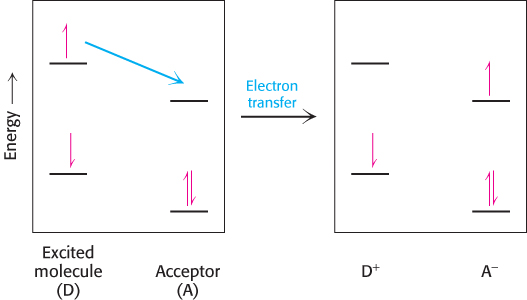
FIGURE 19.8Photoinduced charge separation. If a suitable electron acceptor is nearby, an electron that has been moved to a high energy level by light absorption can move from the excited molecule to the acceptor.
In chloroplasts, the site at which the charge separation takes place within each photosystem is called the reaction center. The photosynthetic apparatus is arranged to maximize photoinduced charge separation and minimize an unproductive return of the electron to its ground state. The electron, extracted from its initial site by the absorption of light, now has reducing power: it can reduce other molecules to store the energy originally obtained from light in chemical forms.
A special pair of chlorophylls initiate charge separation
Photosynthetic bacteria such as Rhodopseudomonas viridis contain a photosynthetic reaction center that has been revealed at atomic resolution. The bacterial reaction center consists of four polypeptides: L (31 kDa, red), M (36 kDa, blue), and H (28 kDa, white) subunits and C, a c-type cytochrome with four c-type hemes (yellow) (Figure 19.9). Sequence comparisons and low-resolution structural studies have revealed that the bacterial reaction center is homologous to the more complex plant systems. Thus, many of our observations of the bacterial system apply to plant systems as well.
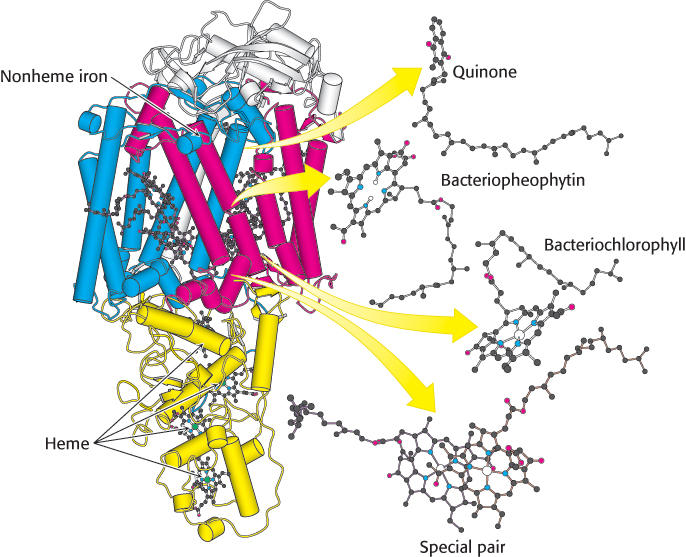
 FIGURE 19.9 Bacterial photosynthetic reaction center. The core of the reaction center from Rhodopseudomonas viridis consists of two similar chains: L (red) and M (blue). An H chain (white) and a cytochrome subunit (yellow) complete the structure. Notice that the L and M subunits are composed largely of α helices that span the membrane. Also notice that a chain of electron-carrying prosthetic groups, beginning with a special pair of bacteriochlorophylls and ending at a bound quinone, runs through the structure from bottom to top in this view.
FIGURE 19.9 Bacterial photosynthetic reaction center. The core of the reaction center from Rhodopseudomonas viridis consists of two similar chains: L (red) and M (blue). An H chain (white) and a cytochrome subunit (yellow) complete the structure. Notice that the L and M subunits are composed largely of α helices that span the membrane. Also notice that a chain of electron-carrying prosthetic groups, beginning with a special pair of bacteriochlorophylls and ending at a bound quinone, runs through the structure from bottom to top in this view.
[Drawn from 1PRC.pdb.]
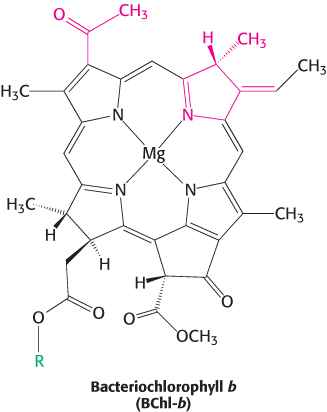
The L and M subunits form the structural and functional core of the bacterial photosynthetic reaction center (Figure 19.9). Each of these homologous subunits contains five transmembrane helices, in contrast with the H subunit, which has just one. The H subunit lies on the cytoplasmic side of the cell membrane, and the cytochrome subunit lies on the exterior face of the cell membrane, called the periplasmic side because it faces the periplasm, the space between the cell membrane and the cell wall. Four bacteriochlorophyll b (BChl-b) molecules, two bacteriopheophytin b (BPh) molecules, two quinones (QA and QB), and a ferrous ion are associated with the L and M subunits.
Bacteriochlorophylls are photoreceptors similar to chlorophylls, except for the reduction of an additional pyrrole ring and other minor differences that shift their absorption maxima to the near infrared, to wavelengths as long as 1000 nm. Bacteriopheophytin is the term for a bacteriochlorophyll that has two protons instead of a magnesium ion at its center.
The reaction begins with light absorption by a pair of BChl-b molecules that lie near the periplasmic side of the membrane in the L–M dimer. The pair of BChl-b molecules is called the special pair because of its fundamental role in photosynthesis. The special pair absorbs light maximally at 960 nm, and, for this reason, is often called P960 (P stands for pigment). After absorbing light, the excited special pair ejects an electron, which is transferred through another BChl-b to a bacteriopheophytin (Figure 19.10, steps 1 and 2). This initial charge separation yields a positive charge on the special pair (P960+) and a negative charge on BPh (BPh−). The electron ejection and transfer take place in less than 10 picoseconds (10−11 s).
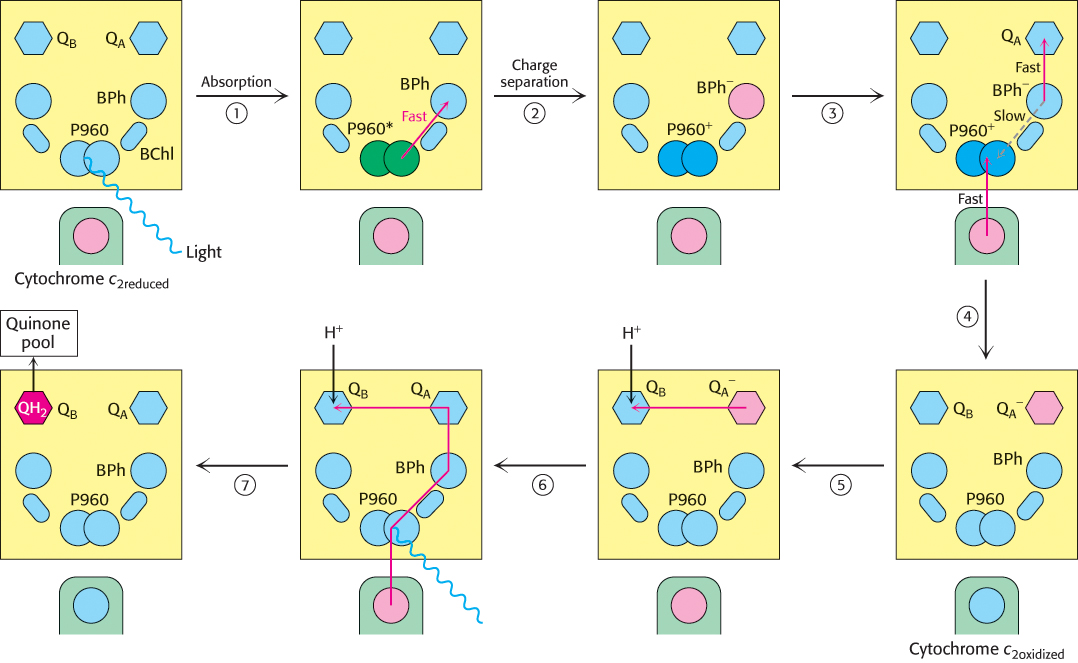
FIGURE 19.10Electron chain in the photosynthetic bacterial reaction center. The absorption of light by the special pair (P960) results in the rapid transfer of an electron from this site to a bacteriopheophytin (BPh), creating a photoinduced charge separation (steps 1 and 2). (The asterisk on P960 stands for excited state.) The possible return of the electron from the pheophytin to the oxidized special pair is suppressed by the “hole” in the special pair being refilled with an electron from the cytochrome subunit and the electron from the pheophytin being transferred to a quinone (QA) that is farther away from the special pair (steps 3 and 4). QA passes the electron to QB. The reduction of a quinone (QB) on the cytoplasmic side of the membrane results in the uptake of two protons from the cytoplasm (steps 5 and 6). The reduced quinone can move into the quinone pool in the membrane (step 7).
A nearby electron acceptor, a tightly bound quinone (QA), quickly grabs the electron away from BPh− before the electron has a chance to fall back to the P960 special pair. From QA, the electron moves to a more loosely associated quinone, QB. The absorption of a second photon and the movement of a second electron from the special pair through the bacteriopheophytin to the quinones completes the two-electron reduction of QB from Q to QH2. Because the QB-binding site lies near the cytoplasmic side of the membrane, two protons are taken up from the cytoplasm, contributing to the development of a proton gradient across the cell membrane (Figure 19.10, steps 5, 6, and 7).
In their high-energy states, P960+ and BPh− could undergo charge recombination; that is, the electron on BPh− could move back to neutralize the positive charge on the special pair. Its return to the special pair would waste a valuable high-energy electron and simply convert the absorbed light energy into heat. How is charge recombination prevented? Two factors in the structure of the reaction center work together to suppress charge recombination nearly completely (Figure 19.10, steps 3 and 4). First, the next electron acceptor (QA) is less than 10 Å away from BPh−, and so the electron is rapidly transferred farther away from the special pair. Second, one of the hemes of the cytochrome subunit is less than 10 Å away from the special pair, and so the positive charge on P960 is neutralized by the transfer of an electron from the reduced cytochrome.
Cyclic electron flow reduces the cytochrome of the reaction center
The cytochrome subunit of the reaction center must regain an electron to complete the cycle. It does so by taking back two electrons from reduced quinone (QH2). QH2 first enters the Q pool in the membrane where it is reoxidized to Q by complex bc1, which is homologous to complex III of the respiratory electron-transport chain. Complex bc1 transfers the electrons from QH2 to cytochrome c2, a water-soluble protein in the periplasm, and in the process pumps protons into the periplasmic space. The electrons now on cytochrome c2 flow to the cytochrome subunit of the reaction center. The flow of electrons is thus cyclic (Figure 19.11). The proton gradient generated in the course of this cycle drives the synthesis of ATP through the action of ATP synthase.
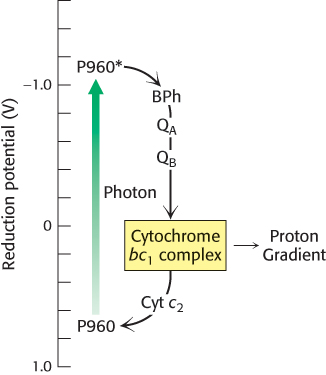
FIGURE 19.11Cyclic electron flow in the bacterial reaction center. Excited electrons from the P960 reaction center flow through bacteriopheophytin (BPh), a pair of quinone molecules (QA and QB), cytochrome bc1 complex, and finally through cytochrome c2 to the reaction center. The cytochrome bc1 complex pumps protons as a result of electron flow, which powers the formation of ATP.





 FIGURE 19.9 Bacterial photosynthetic reaction center. The core of the reaction center from Rhodopseudomonas viridis consists of two similar chains: L (red) and M (blue). An H chain (white) and a cytochrome subunit (yellow) complete the structure. Notice that the L and M subunits are composed largely of α helices that span the membrane. Also notice that a chain of electron-
FIGURE 19.9 Bacterial photosynthetic reaction center. The core of the reaction center from Rhodopseudomonas viridis consists of two similar chains: L (red) and M (blue). An H chain (white) and a cytochrome subunit (yellow) complete the structure. Notice that the L and M subunits are composed largely of α helices that span the membrane. Also notice that a chain of electron-