19.5Accessory Pigments Funnel Energy into Reaction Centers
Accessory Pigments Funnel Energy into Reaction Centers
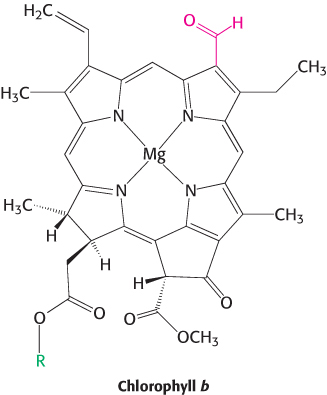
A light-
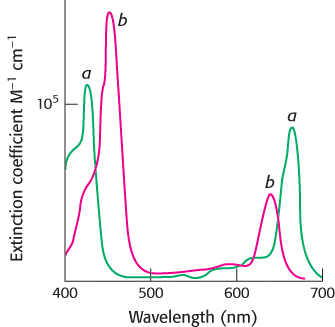
Chlorophyll b and carotenoids are important accessory pigments that funnel energy to the reaction center. Chlorophyll b differs from chlorophyll a in having a formyl group in place of a methyl group. This small difference shifts its two major absorption peaks toward the center of the visible region. In particular, chlorophyll b efficiently absorbs light with wavelengths between 450 and 500 nm (Figure 19.27).
582
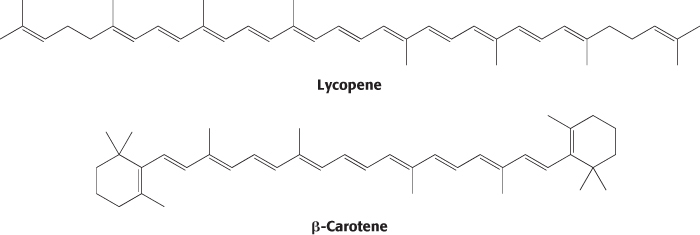
Carotenoids are extended polyenes that absorb light between 400 and 500 nm. The carotenoids are responsible for most of the yellow and red colors of fruits and flowers, and they provide the brilliance of fall, when the chlorophyll molecules degrade, revealing the carotenoids. Tomatoes are especially rich in lycopene, which accounts for their red color, whereas carrots and pumpkins have abundant β-carotene, accounting for their orange color.
In addition to their role in transferring energy to reaction centers, the carotenoids serve a safeguarding function. Carotenoids suppress damaging photochemical reactions, particularly those including oxygen, that can be induced by bright sunlight. This protection may be especially important in the fall, when the primary pigment chlorophyll is being degraded and thus not able to absorb light energy. Plants lacking carotenoids are quickly killed on exposure to light and oxygen.
Resonance energy transfer allows energy to move from the site of initial absorbance to the reaction center
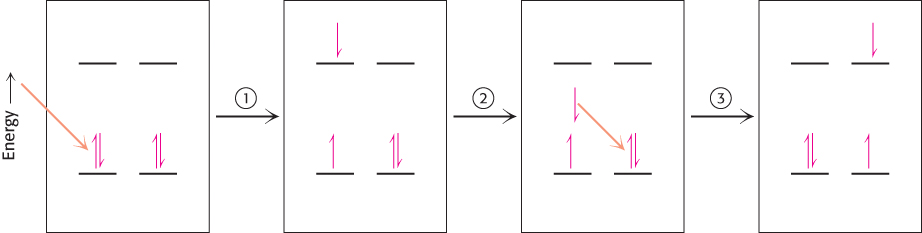
How is energy funneled from accessory pigments to a reaction center? The absorption of a photon does not always lead to electron excitation and transfer. More commonly, excitation energy is transferred from one molecule to a nearby molecule through electromagnetic interactions through space (Figure 19.28). The rate of this process, called resonance energy transfer, depends strongly on the distance between the energy-
583
What is the structural relationship between the accessory pigment and the reaction center? The accessory pigments are arranged in numerous light-
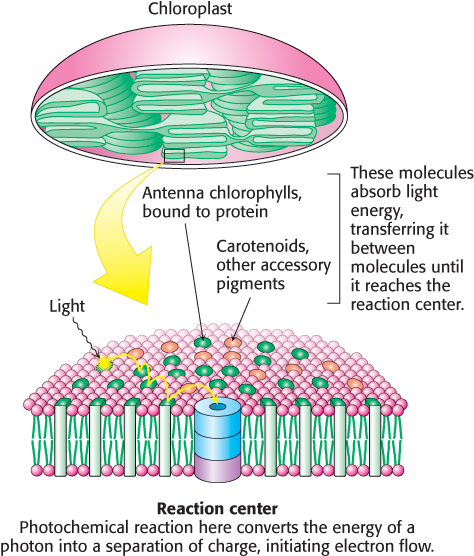
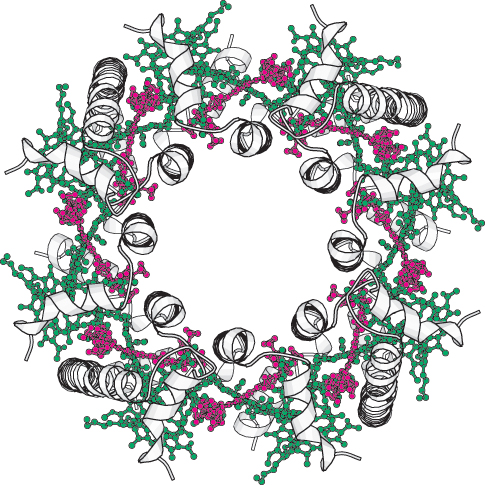
 FIGURE 19.30 Structure of a bacterial light-
FIGURE 19.30 Structure of a bacterial light-The components of photosynthesis are highly organized
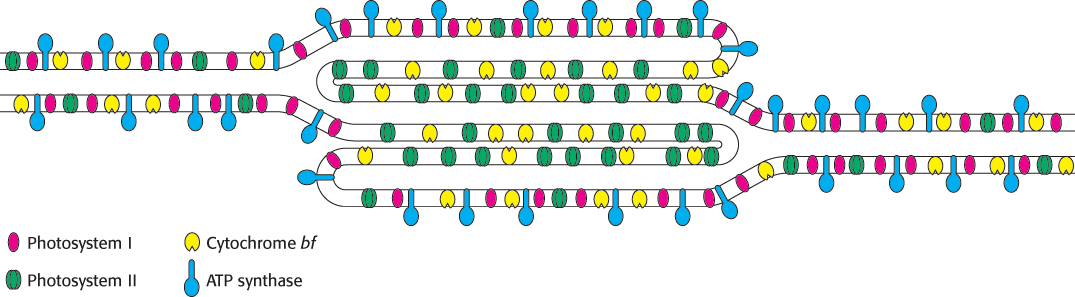
The intricacy of photosynthesis, seen already in the elaborate interplay of complex components, extends even to the placement of the components in the thylakoid membranes. Thylakoid membranes of most plants are differentiated into stacked (appressed) and unstacked (nonappressed) regions (Figures 19.2 and 19.3). Stacking increases the amount of thylakoid membrane in a given chloroplast volume. Both regions surround a common internal thylakoid space, but only unstacked regions make direct contact with the chloroplast stroma. Stacked and unstacked regions differ in the nature of their photosynthetic assemblies (Figure 19.31). Photosystem I and ATP synthase are located almost exclusively in unstacked regions, whereas photosystem II is present mostly in stacked regions. The cytochrome bf complex is found in both regions. Plastoquinone and plastocyanin are the mobile carriers of electrons between assemblies located in different regions of the thylakoid membrane. A common internal thylakoid space enables protons liberated by photosystem II in stacked membranes to be utilized by ATP synthase molecules that are located far away in unstacked membranes.
584
What is the functional significance of this lateral differentiation of the thylakoid membrane system? The positioning of photosystem I in the unstacked membranes also gives it direct access to the stroma for the reduction of NADP+. ATP synthase is also located in the unstacked region to provide space for its large CF1 globule and to give access to ADP. In contrast, the tight quarters of the appressed region pose no problem for photosystem II, which interacts with a small polar electron donor (H2O) and a highly lipid soluble electron carrier (plastoquinone).
Many herbicides inhibit the light reactions of photosynthesis
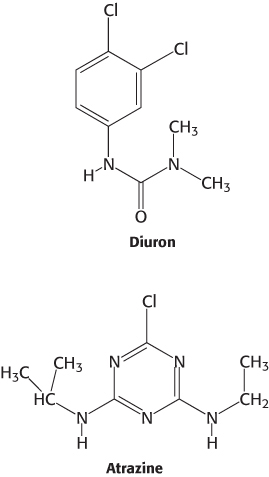
Many commercial herbicides kill weeds by interfering with the action of photosystem II or photosystem I. Inhibitors of photosystem II block electron flow, whereas inhibitors of photosystem I divert electrons from the terminal part of this photosystem. Photosystem II inhibitors include urea derivatives such as diuron and triazine derivatives such as atrazine. These chemicals bind to the QB site of the D1 subunit of photosystem II and block the formation of plastoquinol (QH2).
Paraquat (1,1′-dimethyl-