21.1Glycogen Breakdown Requires the Interplay of Several Enzymes
Glycogen Breakdown Requires the Interplay of Several Enzymes
The efficient breakdown of glycogen to provide glucose 6-
Phosphorylase catalyzes the phosphorolytic cleavage of glycogen to release glucose 1-phosphate
Glycogen phosphorylase, the key enzyme in glycogen breakdown, cleaves its substrate by the addition of orthophosphate (Pi) to yield glucose 1-
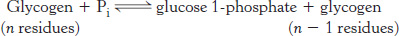
Phosphorylase catalyzes the sequential removal of glucosyl residues from the nonreducing ends of the glycogen molecule (the ends with a free OH group on carbon 4). Orthophosphate splits the glycosidic linkage between C-
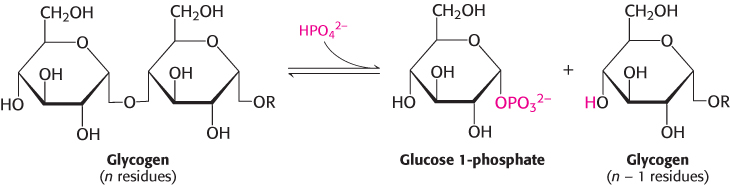
Glucose 1-
620
The reaction catalyzed by phosphorylase is readily reversible in vitro. At pH 6.8, the equilibrium ratio of orthophosphate to glucose 1-
The phosphorolytic cleavage of glycogen is energetically advantageous because the released sugar is already phosphorylated. In contrast, a hydrolytic cleavage would yield glucose, which would then have to be phosphorylated at the expense of a molecule of ATP to enter the glycolytic pathway. An additional advantage of phosphorolytic cleavage for muscle cells is that no transporters exist for glucose 1-
Mechanism: Pyridoxal phosphate participates in the phosphorolytic cleavage of glycogen
The special challenge faced by phosphorylase is to cleave glycogen phosphorolytically rather than hydrolytically to save the ATP required to phosphorylate free glucose. Thus, water must be excluded from the active site. Phosphorylase is a dimer of two identical 97-
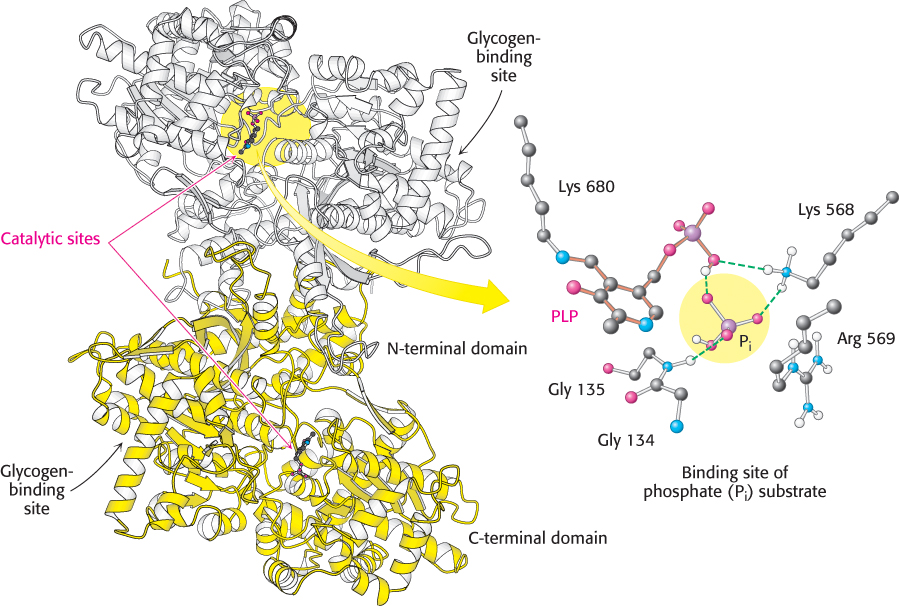
 FIGURE 21.5 Structure of glycogen phosphorylase. This enzyme forms a homodimer: one subunit is shown in white and the other in yellow. Each catalytic site includes a pyridoxal phosphate (PLP) group, linked to lysine 680 of the enzyme. The binding site for the phosphate (Pi) substrate is shown. Notice that the catalytic site lies between the C-
FIGURE 21.5 Structure of glycogen phosphorylase. This enzyme forms a homodimer: one subunit is shown in white and the other in yellow. Each catalytic site includes a pyridoxal phosphate (PLP) group, linked to lysine 680 of the enzyme. The binding site for the phosphate (Pi) substrate is shown. Notice that the catalytic site lies between the C-621
Several clues suggest a mechanism. First, both the glycogen substrate and the glucose 1-
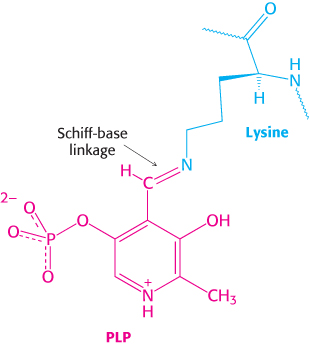
A second clue to the catalytic mechanism of phosphorylase is its requirement for the coenzyme pyridoxal phosphate (PLP), a derivative of pyridoxine (vitamin B6, Section 15.4). The aldehyde group of this coenzyme forms a Schiff-
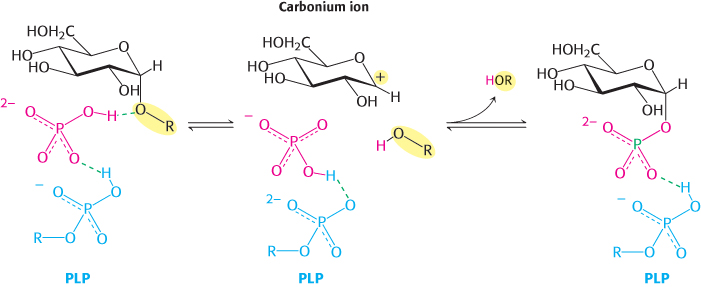
A Schiff base, also called an imine, is a compound containing a carbon–
The glycogen-
A debranching enzyme also is needed for the breakdown of glycogen
Glycogen phosphorylase acting alone degrades glycogen to a limited extent. The enzyme can break α-1,4-
622
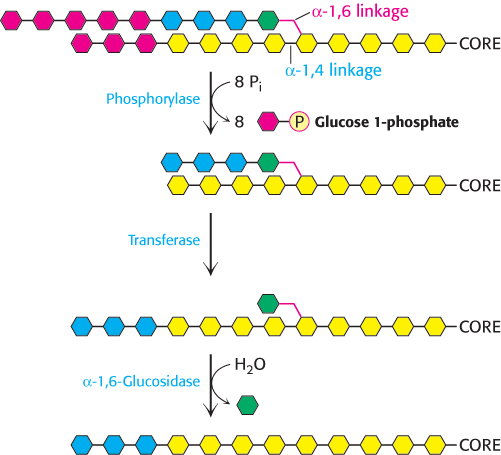
How can the remainder of the glycogen molecule be mobilized for use as a fuel? Two additional enzymes, a transferase and α-1,6-
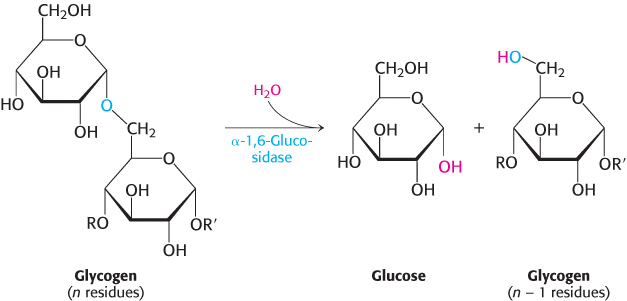
A free glucose molecule is released and then phosphorylated by the glycolytic enzyme hexokinase. Thus, the transferase and α-1,6-
Phosphoglucomutase converts glucose 1-phosphate into glucose 6-phosphate
Glucose 1-
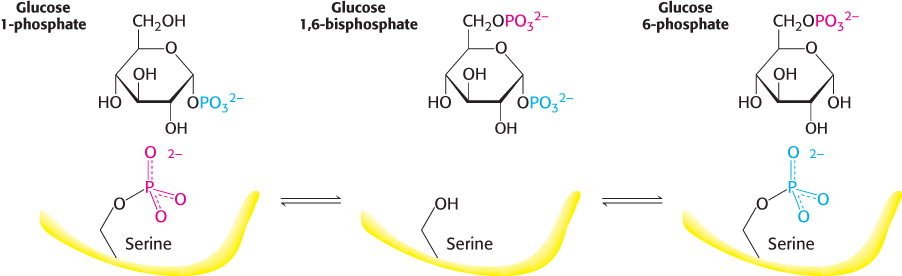
These reactions are like those of phosphoglycerate mutase, a glycolytic enzyme (Section 16.1). The role of glucose 1,6-
The liver contains glucose 6-phosphatase, a hydrolytic enzyme absent from muscle
A major function of the liver is to maintain a nearly constant level of glucose in the blood. The liver releases glucose into the blood between meals and during muscular activity. The released glucose is taken up primarily by the brain, skeletal muscle, and red blood cells. In contrast with unmodified glucose, however, the phosphorylated glucose produced by glycogen breakdown is not transported out of cells. The liver contains a hydrolytic enzyme, glucose 6-
Glucose 6-
623
Glucose 6-