16.1
Glycolysis Is an Energy-Conversion Pathway in Many Organisms
First stage of glycolysis. The first stage of glycolysis begins with the phosphorylation of glucose by hexokinase and ends with the isomerization of dihydroxyacetone phosphate to glyceraldehyde 3-phosphate.
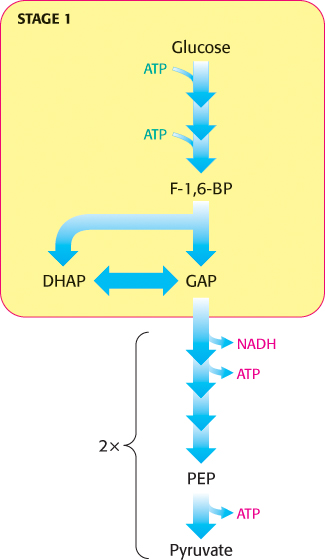
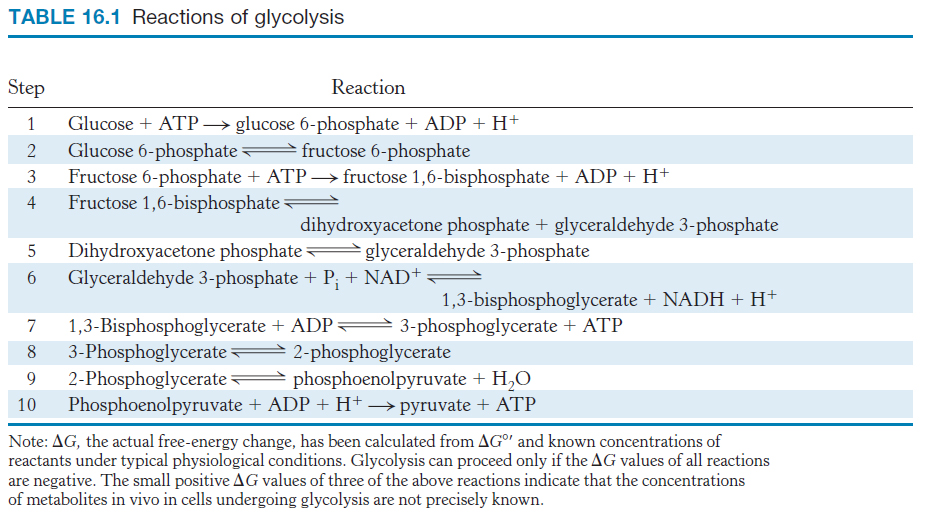
We now begin our consideration of the glycolytic pathway. This pathway is common to virtually all cells, both prokaryotic and eukaryotic. In eukaryotic cells, glycolysis takes place in the cytoplasm. This pathway can be thought of as comprising two stages (Figure 16.2). Stage 1 is the trapping and preparation phase. No ATP is generated in this stage. In stage 1, glucose is converted into fructose 1,6-bisphosphate in three steps: a phosphorylation, an isomerization, and a second phosphorylation reaction. The strategy of these initial steps in glycolysis is to trap the glucose in the cell and form a compound that can be readily cleaved into phosphorylated three-carbon units. Stage 1 is completed with the cleavage of the fructose 1,6-bisphosphate into two three-carbon fragments. These resulting three-carbon units are readily interconvertible. In stage 2, ATP is harvested when the three-carbon fragments are oxidized to pyruvate.
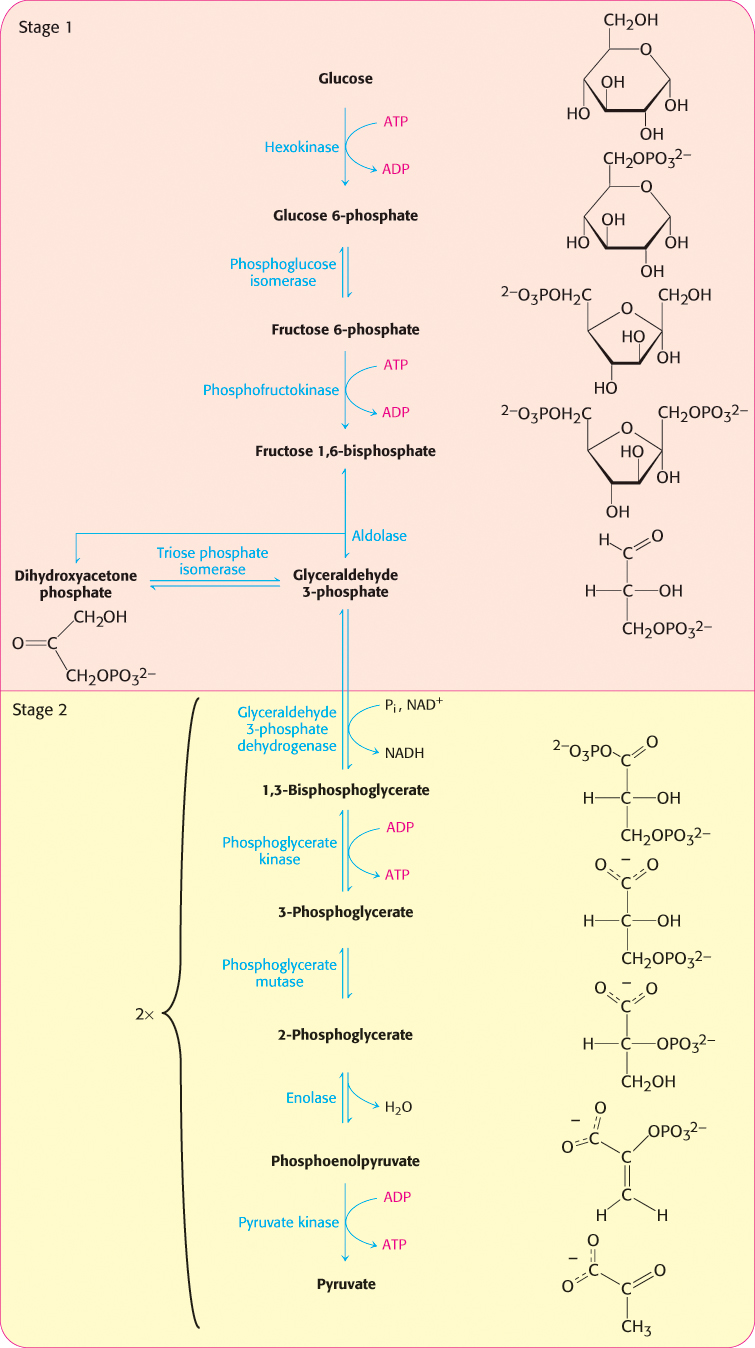
FIGURE 16.2Stages of glycolysis. The glycolytic pathway can be divided into two stages: (1) glucose is trapped, destabilized, and cleaved into two interconvertible three-carbon molecules generated by cleavage of six-carbon fructose; and (2) ATP is generated.
Hexokinase traps glucose in the cell and begins glycolysis
Glucose enters cells through specific transport proteins and has one principal fate: it is phosphorylated by ATP to form glucose 6-phosphate. This step is notable for several reasons. Glucose 6-phosphate cannot pass through the membrane because of the negative charges on the phosphoryl groups, and it is not a substrate for glucose transporters. Also, the addition of the phosphoryl group facilitates the eventual metabolism of glucose into three-carbon molecules with high-phosphoryl-transfer-potential. The transfer of the phosphoryl group from ATP to the hydroxyl group on carbon 6 of glucose is catalyzed by hexokinase.
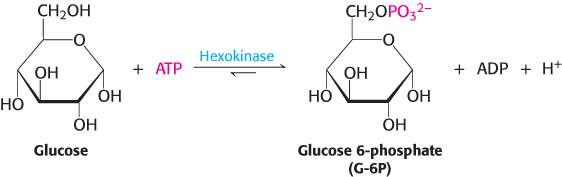
Phosphoryl transfer is a fundamental reaction in biochemistry. Kinases are enzymes that catalyze the transfer of a phosphoryl group from ATP to an acceptor. Hexokinase, then, catalyzes the transfer of a phosphoryl group from ATP to a variety of six-carbon sugars (hexoses), such as glucose and mannose. Hexokinase, like adenylate kinase (Section 9.4) and all other kinases, requires Mg2+ (or another divalent metal ion such as Mn2+) for activity. The divalent metal ion forms a complex with ATP.
X-ray crystallographic studies of yeast hexokinase revealed that the binding of glucose induces a large conformational change in the enzyme. Hexokinase consists of two lobes, which move toward each other when glucose is bound (Figure 16.3). On glucose binding, one lobe rotates 12 degrees with respect to the other, resulting in movements of the polypeptide backbone of as much as 8 Å. The cleft between the lobes closes, and the bound glucose becomes surrounded by protein, except for the hydroxyl group of carbon 6, which will accept the phosphoryl group from ATP. The closing of the cleft in hexokinase is a striking example of the role of induced fit in enzyme action (Section 8.3).
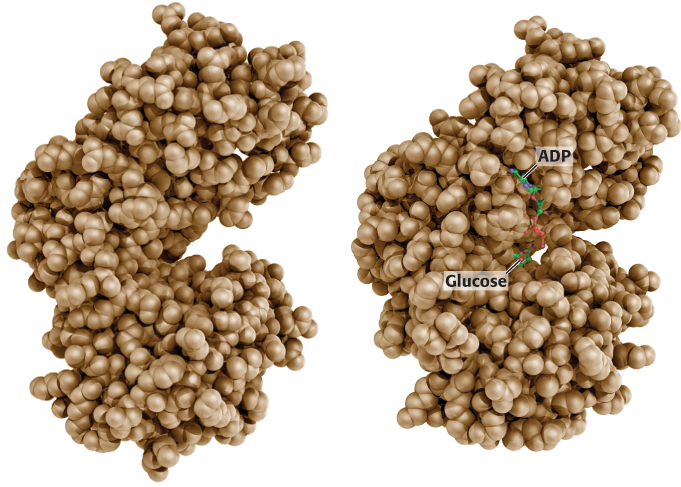
FIGURE 16.3Induced fit in hexokinase. The two lobes of hexokinase are separated in the absence of glucose (left). The conformation of hexokinase changes markedly on binding glucose (right). Notice that two lobes of the enzyme come together, creating the necessary environment for catalysis.
[After RSCB Protein Data Bank; drawn from yhx and 1hkg by Adam Steinberg.]
The glucose-induced structural changes are significant in two respects. First, the environment around the glucose becomes more nonpolar, which favors reaction between the hydrophilic hydroxyl group of glucose and the terminal phosphoryl group of ATP. Second, the conformational changes enable the kinase to discriminate against H2O as a substrate. The closing of the cleft keeps water molecules away from the active site. If hexokinase were rigid, a molecule of H2O occupying the binding site for the —CH2OH of glucose could attack the γ phosphoryl group of ATP, forming ADP and Pi. In other words, a rigid kinase would likely also be an ATPase. It is interesting to note that other kinases taking part in glycolysis—phosphofructokinase, phosphoglycerate kinase, and pyruvate kinase—also contain clefts between lobes that close when substrate is bound, although the structures of these enzymes are different in other regards. Substrate-induced cleft closing is a general feature of kinases. Recall that protein kinase A also undergoes similar structural changes.
Fructose 1,6-bisphosphate is generated from glucose 6-phosphate
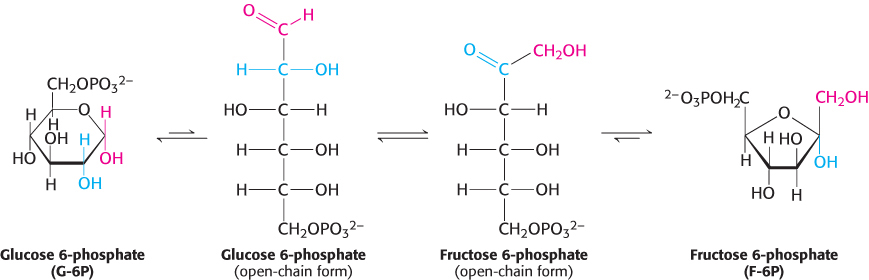
The next step in glycolysis is the isomerization of glucose 6-phosphate to fructose 6-phosphate. Recall that the open-chain form of glucose has an aldehyde group at carbon 1, whereas the open-chain form of fructose has a keto group at carbon 2. Thus, the isomerization of glucose 6-phosphate to fructose 6-phosphate is a conversion of an aldose into a ketose. The reaction catalyzed by phosphoglucose isomerase takes several steps because both glucose 6-phosphate and fructose 6-phosphate are present primarily in the cyclic forms. The enzyme must first open the six-membered ring of glucose 6-phosphate, catalyze the isomerization, and then promote the formation of the five-membered ring of fructose 6-phosphate.
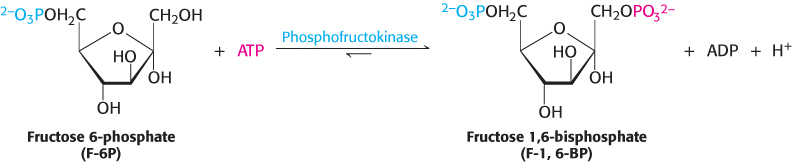
A second phosphorylation reaction follows the isomerization step. Fructose 6-phosphate is phosphorylated at the expense of ATP to fructose 1,6-bisphosphate (F-l,6-BP). The prefix bis- in bisphosphate means that two separate monophosphoryl groups are present, whereas the prefix di- in diphosphate (as in adenosine diphosphate) means that two phosphoryl groups are present and are connected by an anhydride bond.
This reaction is catalyzed by phosphofructokinase (PFK), an allosteric enzyme that sets the pace of glycolysis. As we will learn, this enzyme plays a central role in the metabolism of many molecules in all parts of the body.
What is the biochemical rationale for the isomerization of glucose 6-phosphate to fructose 6-phosphate and its subsequent phosphorylation to form fructose 1,6-bisphosphate? Had the aldol cleavage taken place in the aldose glucose, a two-carbon and a four-carbon fragment would have resulted. Two different metabolic pathways, one to process the two-carbon fragment and one for the four-carbon fragment, would have been required to extract energy. Phosphorylation of the fructose 6-phosphate to fructose 1,6-bisphosphate prevents the reformation of glucose 6-phosphate. As shown below, aldol cleavage of fructose 1,6-bisphosphate yields two phosphorylated interconvertible three-carbon fragments that will be oxidized in the later steps of glycolysis to capture energy in the form of ATP.
The six-carbon sugar is cleaved into two three-carbon fragments
 The newly formed fructose 1,6-bisphosphate is cleaved into glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), completing stage 1 of glycolysis. The products of the remaining steps in glycolysis consist of three-carbon units rather than six-carbon units.
The newly formed fructose 1,6-bisphosphate is cleaved into glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), completing stage 1 of glycolysis. The products of the remaining steps in glycolysis consist of three-carbon units rather than six-carbon units.
This reaction, which is readily reversible, is catalyzed by aldolase. This enzyme derives its name from the nature of the reverse reaction, an aldol condensation.
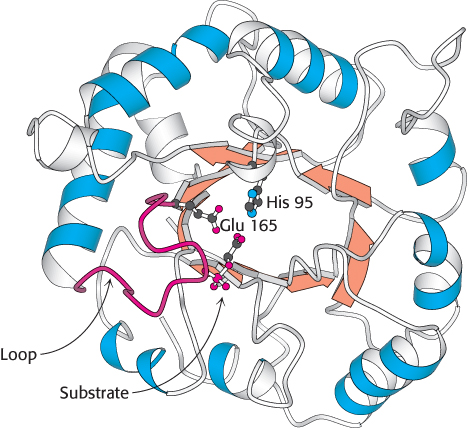
 FIGURE 16.4 Structure of triose phosphate isomerase. This enzyme consists of a central core of eight parallel β strands (orange) surrounded by eight α helices (blue). This structural motif, called an αβ barrel, is also found in the glycolytic enzymes aldolase, enolase, and pyruvate kinase. Notice that histidine 95 and glutamate 165, essential components of the active site of triose phosphate isomerase, are located in the barrel. A loop (red) closes off the active site on substrate binding.
FIGURE 16.4 Structure of triose phosphate isomerase. This enzyme consists of a central core of eight parallel β strands (orange) surrounded by eight α helices (blue). This structural motif, called an αβ barrel, is also found in the glycolytic enzymes aldolase, enolase, and pyruvate kinase. Notice that histidine 95 and glutamate 165, essential components of the active site of triose phosphate isomerase, are located in the barrel. A loop (red) closes off the active site on substrate binding.
[Drawn from 2YPI.pdb.]
Glyceraldehyde 3-phosphate is on the direct pathway of glycolysis, whereas dihydroxyacetone phosphate is not. Unless a means exists to convert dihydroxyacetone phosphate into glyceraldehyde 3-phosphate, a three-carbon fragment useful for generating ATP will be lost. These compounds are isomers that can be readily interconverted: dihydroxyacetone phosphate is a ketose, whereas glyceraldehyde 3-phosphate is an aldose. The isomerization of these three-carbon phosphorylated sugars is catalyzed by triose phosphate isomerase (TPI, sometimes abbreviated TIM; Figure 16.4).
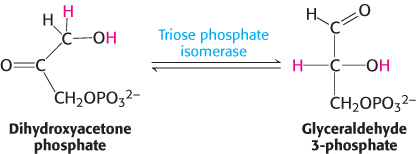
This reaction is rapid and reversible. At equilibrium, 96% of the triose phosphate is dihydroxyacetone phosphate. However, the reaction proceeds readily from dihydroxyacetone phosphate to glyceraldehyde 3-phosphate because the subsequent reactions of glycolysis remove this product. Triose phosphate isomerase deficiency, a rare condition, is the only glycolytic enzymopathy that is lethal. This deficiency is characterized by severe hemolytic anemia and neurodegeneration.
Mechanism: Triose phosphate isomerase salvages a three-carbon fragment
Much is known about the catalytic mechanism of triose phosphate isomerase. TPI catalyzes the transfer of a hydrogen atom from carbon 1 to carbon 2, an intramolecular oxidation–reduction. This isomerization of a ketose into an aldose proceeds through an enediol intermediate (Figure 16.5).
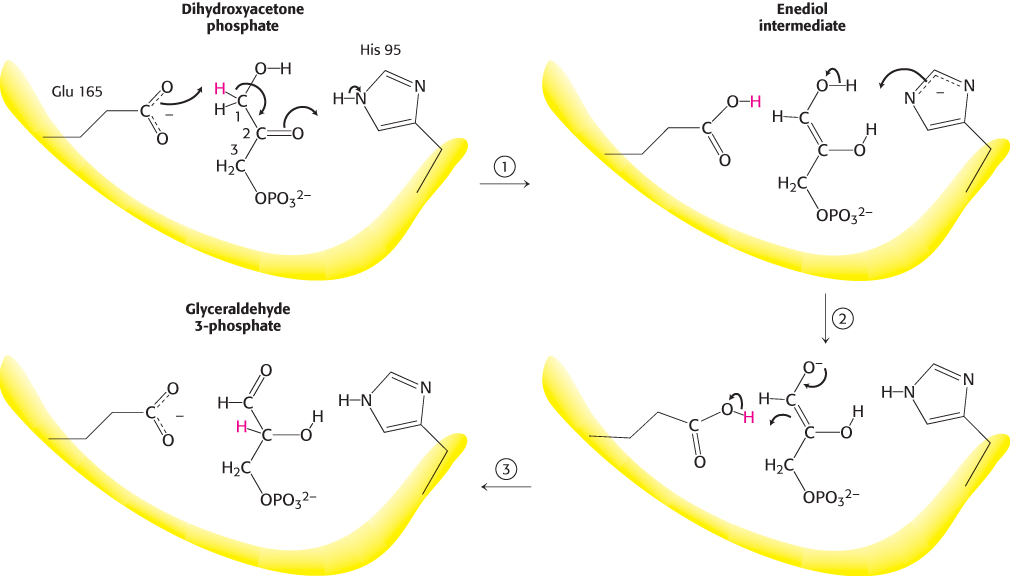
FIGURE 16.5Catalytic mechanism of triose phosphate isomerase. (1) Glutamate 165 acts as a general base by abstracting a proton (H+) from carbon 1. Histidine 95, acting as a general acid, donates a proton to the oxygen atom bonded to carbon 2, forming the enediol intermediate. (2) Glutamic acid, now acting as a general acid, donates a proton to C-2 while histidine removes a proton from the OH group of C-1. (3) The product is formed, and glutamate and histidine are returned to their ionized and neutral forms, respectively.
X-ray crystallographic and other studies showed that glutamate 165 plays the role of a general acid–base catalyst: it abstracts a proton (H+) from carbon 1 and then donates it to carbon 2. However, the carboxylate group of glutamate 165 by itself is not basic enough to pull a proton away from a carbon atom adjacent to a carbonyl group. Histidine 95 assists catalysis by donating a proton to stabilize the negative charge that develops on the C-2 carbonyl group.
Two features of this enzyme are noteworthy. First, TPI displays great catalytic prowess. It accelerates isomerization by a factor of 1010 compared with the rate obtained with a simple base catalyst such as acetate ion. Indeed, the kcat/KM ratio for the isomerization of glyceraldehyde 3-phosphate is 2 × 108 M−1 s−1, which is close to the diffusion-controlled limit. In other words, catalysis takes place every time that enzyme and substrate meet. The diffusion-controlled encounter of substrate and enzyme is thus the rate-limiting step in catalysis. TPI is an example of a kinetically perfect enzyme (Section 8.4). Second, TPI suppresses an undesired side reaction, the decomposition of the enediol intermediate into methyl glyoxal and orthophosphate.
In solution, this physiologically useless reaction is 100 times as fast as isomerization. Moreover, methyl glyoxal is a highly reactive compound that can modify the structure and function of a variety of biomolecules, including proteins and DNA. The reaction of methyl glyoxal with a biomolecule is an example of deleterious reactions called advanced glycation end products, discussed earlier (AGEs, Section 11.1). Hence, TPI must prevent the enediol from leaving the enzyme. This labile intermediate is trapped in the active site by the movement of a loop of 10 residues (Figure 16.4). This loop serves as a lid on the active site, shutting it when the enediol is present and reopening it when isomerization is completed. We see here a striking example of one means of preventing an undesirable alternative reaction: the active site is kept closed until the desirable reaction takes place.
Thus, two molecules of glyceraldehyde 3-phosphate are formed from one molecule of fructose 1,6-bisphosphate by the sequential action of aldolase and triose phosphate isomerase. The economy of metabolism is evident in this reaction sequence. The isomerase funnels dihydroxyacetone phosphate into the main glycolytic pathway; a separate set of reactions is not needed.
The oxidation of an aldehyde to an acid powers the formation of a compound with high phosphoryl-transfer potential
The preceding steps in glycolysis have transformed one molecule of glucose into two molecules of glyceraldehyde 3-phosphate, but no energy has yet been extracted. On the contrary, thus far, two molecules of ATP have been invested. We come now to the second stage of glycolysis, a series of steps that harvest some of the energy contained in glyceraldehyde 3-phosphate as ATP. The initial reaction in this sequence is the conversion of glyceraldehyde 3-phosphate into 1,3-bisphosphoglycerate (1,3-BPG), a reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase.
1,3-Bisphosphoglycerate is an acyl phosphate, which is a mixed anhydride of phosphoric acid and a carboxylic acid. Such compounds have a high phosphoryl-transfer potential; one of its phosphoryl groups is transferred to ADP in the next step in glycolysis.
The reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase can be viewed as the sum of two processes: the oxidation of the aldehyde to a carboxylic acid by NAD+ and the joining of the carboxylic acid and orthophosphate to form the acyl-phosphate product.
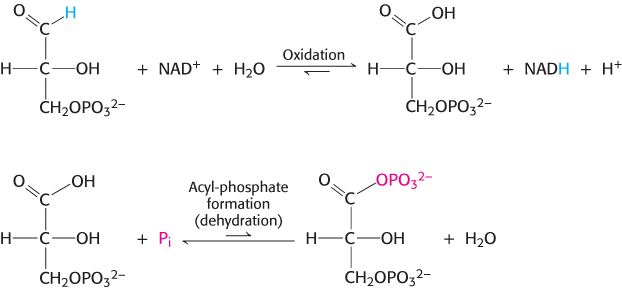
The first reaction is thermodynamically quite favorable, with a standard free-energy change, ΔG°′, of approximately −50 kJ mol−1 (−12 kcal mol−1), whereas the second reaction is quite unfavorable, with a standard free-energy change of the same magnitude but the opposite sign. If these two reactions simply took place in succession, the second reaction would have a very large activation energy and thus not take place at a biologically significant rate. These two processes must be coupled so that the favorable aldehyde oxidation can be used to drive the formation of the acyl phosphate. How are these reactions coupled? The key is an intermediate, formed as a result of the aldehyde oxidation, that is linked to the enzyme by a thioester bond. Thioesters are high-energy compounds found in many biochemical pathways (Section 15.4). This intermediate reacts with orthophosphate to form the high-energy compound 1,3-bisphosphoglycerate.
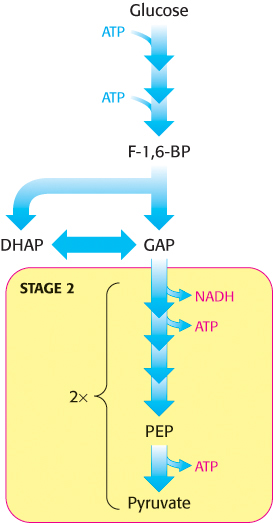
Second stage of glycolysis. The oxidation of three-carbon fragments yields ATP.
The thioester intermediate is higher in free energy than the free carboxylic acid is. The favorable oxidation and unfavorable phosphorylation reactions are coupled by the thioester intermediate, which preserves much of the free energy released in the oxidation reaction. We see here the use of a covalent enzyme-bound intermediate as a mechanism of energy coupling. A free-energy profile of the glyceraldehyde 3-phosphate dehydrogenase reaction, compared with a hypothetical process in which the reaction proceeds without this intermediate, reveals how this intermediate allows a favorable process to drive an unfavorable one (Figure 16.6).
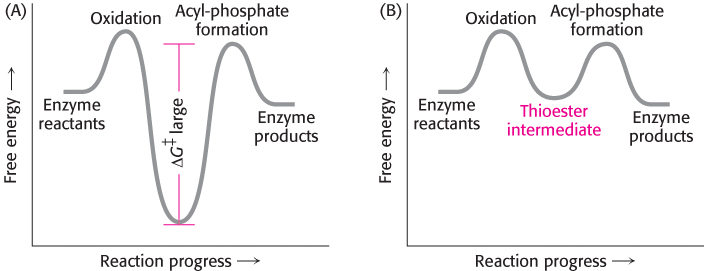
FIGURE 16.6Free-energy profiles for glyceraldehyde oxidation followed by acyl-phosphate formation. (A) A hypothetical case with no coupling between the two processes. The second step must have a large activation barrier, making the reaction very slow. (B) The actual case with the two reactions coupled through a thioester intermediate.
Mechanism: Phosphorylation is coupled to the oxidation of glyceraldehyde 3-phosphate by a thioester intermediate
The active site of glyceraldehyde 3-phosphate dehydrogenase includes a reactive cysteine residue, as well as NAD+ and a crucial histidine (Figure 16.7). Let us consider in detail how these components cooperate in the reaction mechanism (Figure 16.8). In step 1, the aldehyde substrate reacts with the sulfhydryl group of cysteine 149 on the enzyme to form a hemithioacetal. Step 2 is the transfer of a hydride ion to a molecule of NAD+ that is tightly bound to the enzyme and is adjacent to the cysteine residue. This reaction is favored by the deprotonation of the hemithioacetal by histidine 176. The products of this reaction are the reduced coenzyme NADH and a thioester intermediate. This thioester intermediate has a free energy close to that of the reactants (Figure 16.6). In step 3, the NADH formed from the aldehyde oxidation leaves the enzyme and is replaced by a second molecule of NAD+. This step is important because the positive charge on NAD+ polarizes the thioester intermediate to facilitate the attack by orthophosphate. In step 4, orthophosphate attacks the thioester to form 1,3-BPG and free the cysteine residue. This example illustrates the essence of energy transformations and of metabolism itself: energy released by carbon oxidation is converted into high phosphoryl-transfer potential.
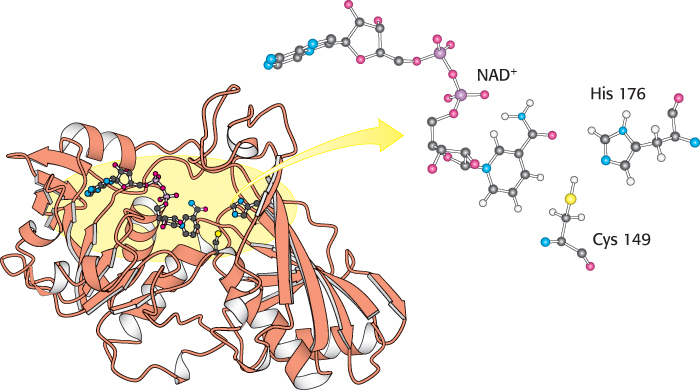
 FIGURE 16.7 Structure of glyceraldehyde 3-phosphate dehydrogenase. Notice that the active site includes a cysteine residue and a histidine residue adjacent to a bound NAD+ molecule. The sulfur atom of cysteine will link with the substrate to form a transitory thioester intermediate.
FIGURE 16.7 Structure of glyceraldehyde 3-phosphate dehydrogenase. Notice that the active site includes a cysteine residue and a histidine residue adjacent to a bound NAD+ molecule. The sulfur atom of cysteine will link with the substrate to form a transitory thioester intermediate.
[Drawn from 1GAD.pdb.]
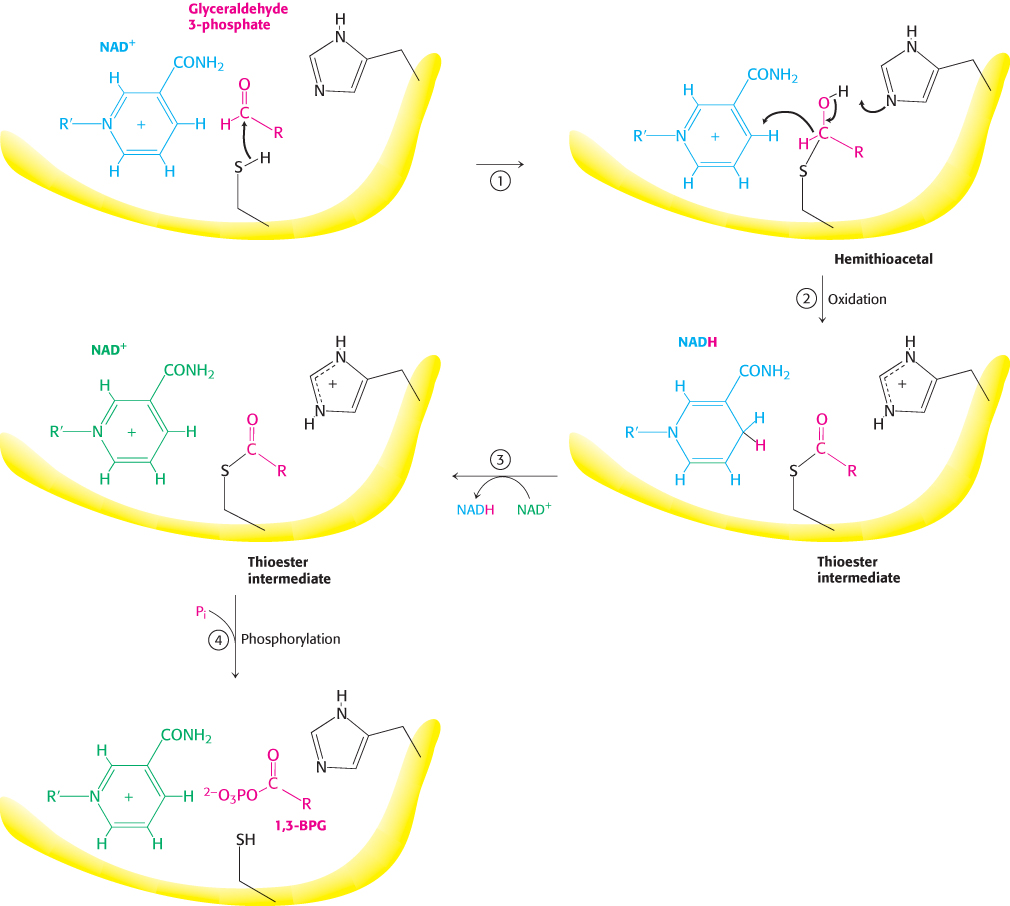
FIGURE 16.8Catalytic mechanism of glyceraldehyde 3-phosphate dehydrogenase. The reaction proceeds through a thioester intermediate, which allows the oxidation of glyceraldehyde to be coupled to the phosphorylation of 3-phosphoglycerate. (1) Cysteine reacts with the aldehyde group of the substrate, forming a hemithioacetal. (2) An oxidation takes place with the transfer of a hydride ion to NAD+, forming a thioester. This reaction is facilitated by the transfer of a proton to histidine. (3) The reduced NADH is exchanged for an NAD+ molecule. (4) Orthophosphate attacks the thioester, forming the product 1,3-BPG.
ATP is formed by phosphoryl transfer from 1,3-bisphosphoglycerate
1,3-Bisphosphoglycerate is an energy-rich molecule with a greater phosphoryl-transfer potential than that of ATP (Section 15.2). Thus, 1,3-BPG can be used to power the synthesis of ATP from ADP. Phosphoglycerate kinase catalyzes the transfer of the phosphoryl group from the acyl phosphate of 1,3-bisphosphoglycerate to ADP. ATP and 3-phosphoglycerate are the products.
The formation of ATP in this manner is referred to as substrate-level phosphorylation because the phosphate donor, 1,3-BPG, is a substrate with high phosphoryl-transfer potential. We will contrast this manner of ATP formation with the formation of ATP from ionic gradients in chapters 18 and 19.
Thus, the outcomes of the reactions catalyzed by glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase are as follows:
Glyceraldehyde 3-phosphate, an aldehyde, is oxidized to 3-phosphoglycerate, a carboxylic acid.
NAD+ is concomitantly reduced to NADH.
ATP is formed from Pi and ADP at the expense of carbon-oxidation energy.
In essence, the energy released during the oxidation of glyceraldehyde 3-phosphate to 3-phosphoglycerate is temporarily trapped as 1,3-bisphosphoglycerate. This energy powers the transfer of a phosphoryl group from 1,3-bisphosphoglycerate to ADP to yield ATP. Keep in mind that, because of the actions of aldolase and triose phosphate isomerase, two molecules of glyceraldehyde 3-phosphate were formed and hence two molecules of ATP were generated. These ATP molecules make up for the two molecules of ATP consumed in the first stage of glycolysis.
Additional ATP is generated with the formation of pyruvate
In the remaining steps of glycolysis, 3-phosphoglycerate is converted into pyruvate, and a second molecule of ATP is formed from ADP.
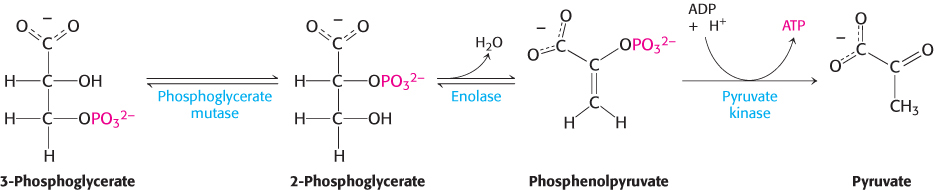
The first reaction is a rearrangement. The position of the phosphoryl group shifts in the conversion of 3-phosphoglycerate into 2-phosphoglycerate, a reaction catalyzed by phosphoglycerate mutase. In general, a mutase is an enzyme that catalyzes the intramolecular shift of a chemical group, such as a phosphoryl group. The phosphoglycerate mutase reaction has an interesting mechanism: the phosphoryl group is not simply moved from one carbon atom to another. This enzyme requires catalytic amounts of 2,3-bisphosphoglycerate (2,3-BPG) to maintain an active-site histidine residue in a phosphorylated form. This phosphoryl group is transferred to 3-phosphoglycerate to reform 2,3-bisphosphoglycerate.
The mutase then functions as a phosphatase: it converts 2,3-bisphosphoglycerate into 2-phosphoglycerate. The mutase retains the phosphoryl group to regenerate the modified histidine.
The sum of these reactions yields the mutase reaction:
In the next reaction, the dehydration of 2-phosphoglycerate introduces a double bond, creating an enol. Enolase catalyzes this formation of the enol phosphate phosphoenolpyruvate (PEP). This dehydration markedly elevates the transfer potential of the phosphoryl group. An enol phosphate has a high phosphoryl-transfer potential, whereas the phosphate ester of an ordinary alcohol, such as 2-phosphoglycerate, has a low one. The ΔG°′ of the hydrolysis of a phosphate ester of an ordinary alcohol is −13 kJ mol−1 (−3 kcal mol−1), whereas that of phosphoenolpyruvate is −62 kJ mol−1 (−15 kcal mol−1).
Why does phosphoenolpyruvate have such a high phosphoryl-transfer potential? The phosphoryl group traps the molecule in its unstable enol form. When the phosphoryl group has been donated to ATP, the enol undergoes a conversion into the more stable ketone—namely, pyruvate.
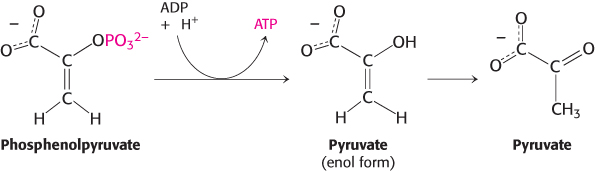
Thus, the high phosphoryl-transfer potential of phosphoenolpyruvate arises primarily from the large driving force of the subsequent enol–ketone conversion. Hence, pyruvate is formed, and ATP is generated concomitantly. The virtually irreversible transfer of a phosphoryl group from phosphoenolpyruvate to ADP is catalyzed by pyruvate kinase. What is the energy source for the formation of phosphoenolpyruvate? The answer to this question becomes clear when we compare the structures of 2-phosphoglycerate and pyruvate. The formation of pyruvate from 2-phosphoglycerate is, in essence, an internal oxidation–reduction reaction; carbon 3 takes electrons from carbon 2 in the conversion of 2-phosphoglycerate into pyruvate. Compared with 2-phosphoglycerate, C-3 is more reduced in pyruvate, whereas C-2 is more oxidized. Once again, carbon oxidation powers the synthesis of a compound with high phosphoryl-transfer potential, phos-phoenolpyruvate here and 1,3-bisphosphoglycerate earlier, which allows the synthesis of ATP.
Because the molecules of ATP used in forming fructose 1,6-bisphosphate have already been regenerated, the two molecules of ATP generated from phosphoenolpyruvate are “profit.”
NAD+ is regenerated from the metabolism of pyruvate
The conversion of glucose into two molecules of pyruvate has resulted in the net synthesis of ATP. However, an energy-converting pathway that stops at pyruvate will not proceed for long, because redox balance has not been maintained. As we have seen, the activity of glyceraldehyde 3-phosphate dehydrogenase, in addition to generating a compound with high phosphoryl-transfer potential, reduces NAD+ to NADH. In the cell, there are limited amounts of NAD+, which is derived from the vitamin niacin (B3), a dietary requirement for human beings. Consequently, NAD+ must be regenerated for glycolysis to proceed. Thus, the final process in the pathway is the regeneration of NAD+ through the metabolism of pyruvate.
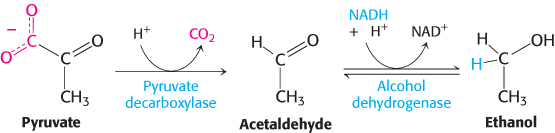
The sequence of reactions from glucose to pyruvate is similar in most organisms and most types of cells. In contrast, the fate of pyruvate is variable. Three reactions of pyruvate are of primary importance: conversion into ethanol, lactate, or carbon dioxide (Figure 16.9). The first two reactions are fermentations that take place in the absence of oxygen. A fermentation is an ATP-generating process in which organic compounds act both as the donor and as the acceptor of electrons. In the presence of oxygen, the most common situation in multicellular organisms and in many unicellular ones, pyruvate is metabolized to carbon dioxide and water through the citric acid cycle and the electron-transport chain with oxygen serving as the final electron acceptor. We now take a closer look at these three possible fates of pyruvate.
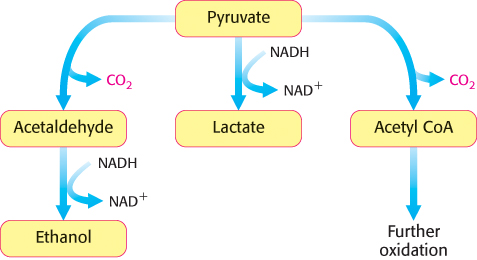
FIGURE 16.9Diverse fates of pyruvate. Ethanol and lactate can be formed by reactions that include NADH. Alternatively, a two-carbon unit from pyruvate can be coupled to coenzyme A (Chapter 17) to form acetyl CoA.
1. Ethanol is formed from pyruvate in yeast and several other microorganisms. The first step is the decarboxylation of pyruvate. This reaction is catalyzed by pyruvate decar-boxylase, which requires the coenzyme thiamine pyrophosphate, a coenzyme, derived from the vitamin thiamine (B1). The second step is the reduction of acetaldehyde to ethanol by NADH, in a reaction catalyzed by alcohol dehydrogenase. This reaction regenerates NAD+.
|
|
|
ΔG°′ in kJ mol−1 (kcal mol−1) |
ΔG in kJ mol−1 (kcal mol−1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Triose phosphate isomerase |
|
|
|
Glyceraldehyde 3-phosphate dehydrogenase |
Phosphorylation coupled to oxidation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The active site of alcohol dehydrogenase contains a zinc ion that is coordinated to the sulfur atoms of two cysteine residues and a nitrogen atom of histidine (Figure 16.10). This zinc ion polarizes the carbonyl group of the substrate to favor the transfer of a hydride from NADH.
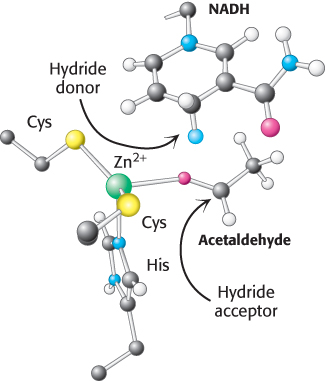
FIGURE 16.10Active site of alcohol dehydrogenase. The active site contains a zinc ion bound to two cysteine residues and one histidine residue. Notice that the zinc ion binds the acetaldehyde substrate through its oxygen atom, polarizing the substrate so that it more easily accepts a hydride from NADH. Only the nicotinamide ring of NADH is shown.
The conversion of glucose into ethanol is an example of alcoholic fermentation. The net result of this anaerobic process is
Note that NAD+ and NADH do not appear in this equation, even though they are crucial for the overall process. NADH generated by the oxidation of glyceraldehyde 3-phosphate is consumed in the reduction of acetaldehyde to ethanol. Thus, there is no net oxidation–reduction in the conversion of glucose into ethanol (Figure 16.11). The ethanol formed in alcoholic fermentation provides a key ingredient for brewing and winemaking.
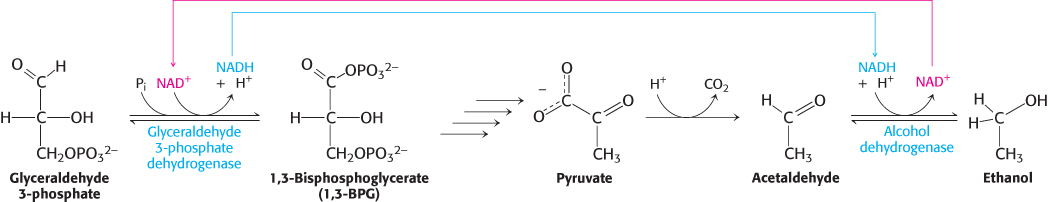
FIGURE 16.11Maintaining redox balance. The NADH produced by the glyceraldehyde 3-phosphate dehydrogenase reaction must be reoxidized to NAD+ for the glycolytic pathway to continue. In alcoholic fermentation, alcohol dehydrogenase oxidizes NADH and generates ethanol. In lactic acid fermentation (not shown), lactate dehydrogenase oxidizes NADH while generating lactic acid.
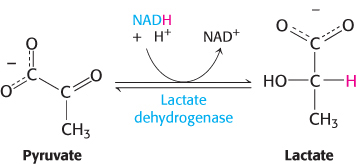
2. Lactate is formed from pyruvate in a variety of microorganisms in a process called lactic acid fermentation. Certain types of skeletal muscles in most animals can also function anaerobically for short periods. For example, a specific type of muscle fiber, called fast twitch or type IIb fibers, performs short bursts of intense exercise. The ATP needs rise faster than the ability of the body to provide oxygen to the muscle. The muscle functions anaerobically until fatigue sets in, which is caused, in part, by lactate buildup. Indeed, the pH of resting type IIb muscle fibers, which is about 7.0, may fall to as low as 6.3 during the bout of exercise. The drop in pH inhibits phosphofructokinase. A lactate/H+ symporter allows the exit of lactate from the muscle cell. The reduction of pyruvate by NADH to form lactate is catalyzed by lactate dehydrogenase.
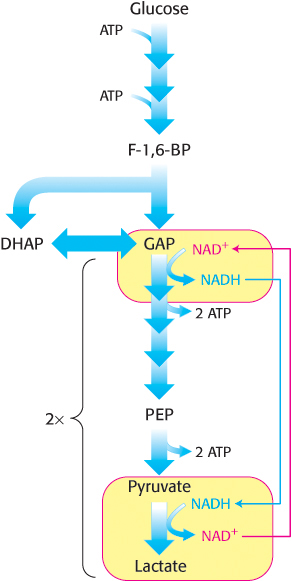
Regeneration of NAD+.
The overall reaction in the conversion of glucose into lactate is
Glucose + 2 Pi + 2 ADP → 2 lactate + 2 ATP + 2 H2O
As in alcoholic fermentation, there is no net oxidation–reduction. The NADH formed in the oxidation of glyceraldehyde 3-phosphate is consumed in the reduction of pyruvate. The regeneration of NAD+ in the reduction of pyruvate to lactate or ethanol sustains the continued process of glycolysis under anaerobic conditions.
3. Only a fraction of the energy of glucose is released in its anaerobic conversion into ethanol or lactate. Much more energy can be extracted aerobically by means of the citric acid cycle and the electron-transport chain. The entry point to this oxidative pathway is acetyl coenzyme A (acetyl CoA), which is formed inside mitochondria by the oxidative decarboxylation of pyruvate.
Pyruvate + NAD+ + CoA → acetyl CoA + CO2 + NADH + H+
This reaction, which is catalyzed by the pyruvate dehydrogenase complex, will be considered in detail in Chapter 17. The NAD+ required for this reaction and for the oxidation of glyceraldehyde 3-phosphate is regenerated when NADH ultimately transfers its electrons to O2 through the electron-transport chain in mitochondria.
Fermentations provide usable energy in the absence of oxygen
TABLE 16.3 Starting and ending points of various fermentations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: The products of some fermentations are the substrates for others. |
Fermentations yield only a fraction of the energy available from the complete combustion of glucose. Why is a relatively inefficient metabolic pathway so extensively used? The fundamental reason is that oxygen is not required. The ability to survive without oxygen affords a host of living accommodations such as soils, deep water, and skin pores. Some organisms, called obligate anaerobes, cannot survive in the presence of O2, a highly reactive compound. The bacterium Clostridium perfringens, the cause of gangrene, is an example of an obligate anaerobe. Other pathogenic obligate anaerobes are listed in Table 16.2. Some organisms, such as yeast, are facultative anaerobes that metabolize glucose aerobically when oxygen is present and perform fermentation when oxygen is absent.
TABLE 16.2 Examples of pathogenic obligate anaerobes
|
|
|
|
|
|
|
|
Botulism (an especially severe type of food poisoning) |
|
|
Gas gangrene (gas is produced as an end point of the fermentation, distorting and destroying the tissue) |
|
|
Cat scratch fever (flu-like symptoms) |
|
|
Abdominal, pelvic, pulmonary, and blood infections |
Although we have considered only lactic acid and alcoholic fermentation, microorganisms are capable of generating a wide array of molecules as end points of fermentation (Table 16.3). Indeed, many food products, including sour cream, yogurt, various cheeses, beer, wine, and sauerkraut, result from fermentation.
The binding site for NAD+ is similar in many dehydrogenases
 The three dehydrogenases—glyceraldehyde 3-phosphate dehydrogenase, alcohol dehydrogenase, and lactate dehydrogenase—have quite different three-dimensional structures. However, their NAD+-binding domains are strikingly similar (Figure 16.12). This nucleotide-binding region is made up of four α helices and a sheet of six parallel β strands. Moreover, in all cases, the bound NAD+ displays nearly the same conformation. This common structural domain is often called a Rossmann fold after Michael Rossmann, who first recognized it. This fold likely represents a primordial dinucleotide-binding domain that recurs in the dehydrogenases of glycolysis and other enzymes because of their descent from a common ancestor.
The three dehydrogenases—glyceraldehyde 3-phosphate dehydrogenase, alcohol dehydrogenase, and lactate dehydrogenase—have quite different three-dimensional structures. However, their NAD+-binding domains are strikingly similar (Figure 16.12). This nucleotide-binding region is made up of four α helices and a sheet of six parallel β strands. Moreover, in all cases, the bound NAD+ displays nearly the same conformation. This common structural domain is often called a Rossmann fold after Michael Rossmann, who first recognized it. This fold likely represents a primordial dinucleotide-binding domain that recurs in the dehydrogenases of glycolysis and other enzymes because of their descent from a common ancestor.
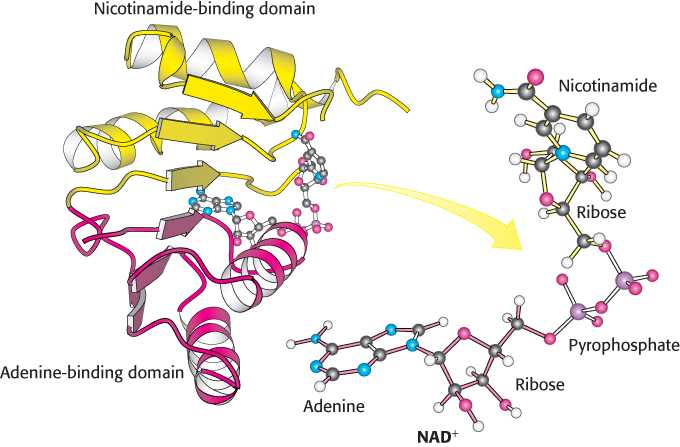
FIGURE 16.12NAD+-binding region in dehydrogenases. Notice that the nicotinamide-binding half (yellow) is structurally similar to the adenine-binding half (red). The two domains together form a structural motif called a Rossmann fold. The NAD+ molecule binds in an extended conformation.
[Drawn from 3LDH.pdb.]
Fructose is converted into glycolytic intermediates by fructokinase
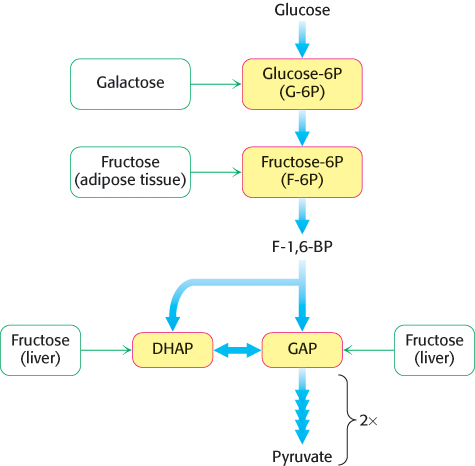
FIGURE 16.13Entry points in glycolysis for fructose and galactose.
Although glucose is the most widely used monosaccharide, others also are important fuels. Let us consider how fructose is funneled into the glycolytic pathway (Figure 16.13). There are no catabolic pathways for metabolizing fructose, and so the strategy is to convert this sugar into a metabolite of glucose.
The main site of fructose metabolism is the liver, using the fructose 1-phosphate pathway (Figure 16.14). The first step is the phosphorylation of fructose to fructose 1-phosphate by fructokinase. Fructose 1-phosphate is then split into glyceraldehyde and dihydroxyacetone phosphate, an intermediate in glycolysis. This aldol cleavage is catalyzed by a specific fructose 1-phosphate aldolase. Glyceraldehyde is then phosphorylated to glyceraldehyde 3-phosphate, a glycolytic intermediate, by triose kinase. In other tissues, such as adipose tissue, fructose can be phosphorylated to fructose 6-phosphate by hexokinase.
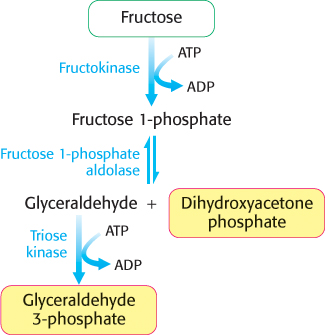
FIGURE 16.14Fructose metabolism. Fructose enters the glycolytic pathway in the liver through the fructose 1-phosphate pathway.
Excessive fructose consumption can lead to pathological conditions
 Fructose, a commonly used sweetener, is a component of sucrose and high fructose corn syrup (which contains approximately 55% fructose and 45% glucose). Epidemiological as well as clinical studies have linked excessive fructose consumption to fatty liver, insulin insensitivity, and obesity. These conditions may eventually result in type 2 diabetes (Chapter 27). Studies have shown that these disorders are not necessarily the result of simple excess energy consumption, but rather how fructose is processed by the liver. What aspects of liver fructose metabolism are the contributing factors then? Note that, as shown in Figure 16.14, the actions of fructokinase and triose kinase bypass the most important regulatory step in glycolysis, the phosphofructokinase-catalyzed reaction. The fructose-derived glyceraldehyde 3-phosphate and dihydroxyacetone phosphate are processed by glycolysis to pyruvate and subsequently to acetyl CoA in an unregulated fashion. As we will see in Chapter 22, this excess acetyl CoA is converted to fatty acids, which can be transported to adipose tissue, resulting in obesity. The liver also begins to accumulate fatty acids, resulting in fatty liver. The activity of the fructokinase and triose kinase can deplete the liver of ATP and inorganic phosphate, compromising liver function. We will return to the topic of obesity and caloric homeostasis in Chapter 27.
Fructose, a commonly used sweetener, is a component of sucrose and high fructose corn syrup (which contains approximately 55% fructose and 45% glucose). Epidemiological as well as clinical studies have linked excessive fructose consumption to fatty liver, insulin insensitivity, and obesity. These conditions may eventually result in type 2 diabetes (Chapter 27). Studies have shown that these disorders are not necessarily the result of simple excess energy consumption, but rather how fructose is processed by the liver. What aspects of liver fructose metabolism are the contributing factors then? Note that, as shown in Figure 16.14, the actions of fructokinase and triose kinase bypass the most important regulatory step in glycolysis, the phosphofructokinase-catalyzed reaction. The fructose-derived glyceraldehyde 3-phosphate and dihydroxyacetone phosphate are processed by glycolysis to pyruvate and subsequently to acetyl CoA in an unregulated fashion. As we will see in Chapter 22, this excess acetyl CoA is converted to fatty acids, which can be transported to adipose tissue, resulting in obesity. The liver also begins to accumulate fatty acids, resulting in fatty liver. The activity of the fructokinase and triose kinase can deplete the liver of ATP and inorganic phosphate, compromising liver function. We will return to the topic of obesity and caloric homeostasis in Chapter 27.
Galactose is converted into glucose 6-phosphate
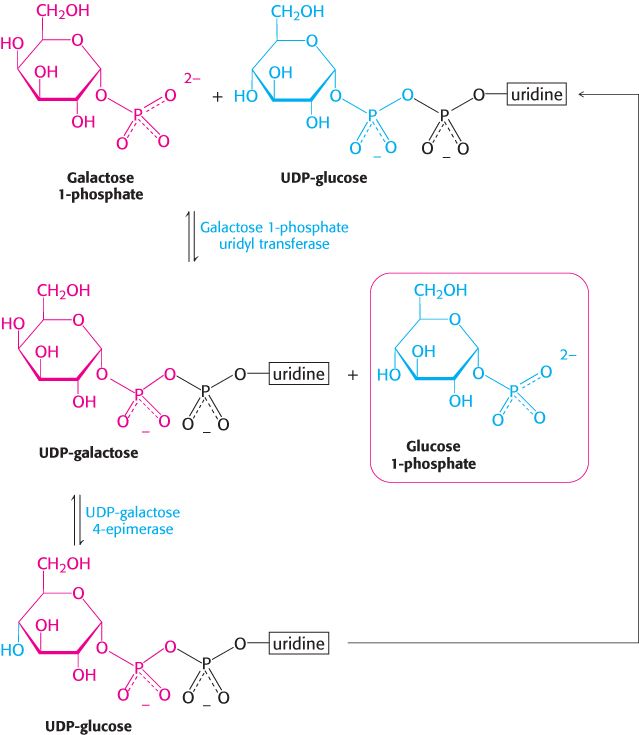
Like fructose, galactose is an abundant sugar that must be converted into metabolites of glucose (Figure 16.13). Galactose is converted into glucose 6-phosphate in four steps. The first reaction in the galactose–glucose interconversion pathway is the phosphorylation of galactose to galactose 1-phosphate by galactokinase.
Galactose 1-phosphate then acquires a uridyl group from uridine diphosphate glucose (UDP-glucose), an activated intermediate in the synthesis of carbohydrates (Section 21.4).
The products of this reaction, which is catalyzed by galactose 1-phosphate uridyl transferase, are UDP-galactose and glucose 1-phosphate. The galactose moiety of UDP-galactose is then epimerized to glucose. The configuration of the hydroxyl group at carbon 4 is inverted by UDP-galactose 4-epimerase.
The sum of the reactions catalyzed by galactokinase, the transferase, and the epimerase is
Galactose + ATP → glucose 1-phosphate + ADP + H+
Note that UDP-glucose is not consumed in the conversion of galactose into glucose, because it is regenerated from UDP-galactose by the epimerase. This reaction is reversible, and the product of the reverse direction also is important. The conversion of UDP-glucose into UDP-galactose is essential for the synthesis of galactosyl residues in complex polysaccharides and glycoproteins if the amount of galactose in the diet is inadequate to meet these needs.
Finally, glucose 1-phosphate, formed from galactose, is isomerized to glucose 6-phosphate by phosphoglucomutase.
We shall return to this reaction when we consider the synthesis and degradation of glycogen, which proceeds through glucose 1-phosphate, in Chapter 21.
Many adults are intolerant of milk because they are deficient in lactase
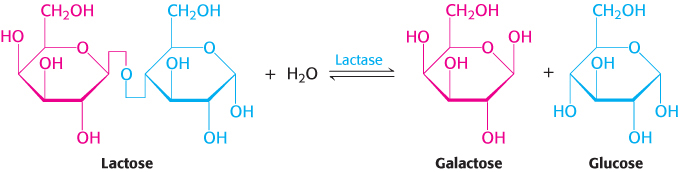
 Many adults are unable to metabolize the milk sugar lactose and experience gastrointestinal disturbances if they drink milk. Lactose intolerance, or hypolactasia, is most commonly caused by a deficiency of the enzyme lactase, which cleaves lactose into glucose and galactose.
Many adults are unable to metabolize the milk sugar lactose and experience gastrointestinal disturbances if they drink milk. Lactose intolerance, or hypolactasia, is most commonly caused by a deficiency of the enzyme lactase, which cleaves lactose into glucose and galactose.
Scanning electron micrograph of Lactobacillus. The anaerobic bacterium Lactobacillus is shown here. As suggested by its name, this genus of bacteria ferments glucose into lactic acid and is widely used in the food industry. Lactobacillus is also a component of the normal human bacterial flora of the urogenital tract where, because of its ability to generate an acidic environment, it prevents the growth of harmful bacteria.
[Power and Syred/Science Photo Library.]
“Deficiency” is not quite the appropriate term, because a decrease in lactase is normal in the course of development in all mammals. As children are weaned and milk becomes less prominent in their diets, lactase activity normally declines to about 5% to 10% of the level at birth. This decrease is not as pronounced with some groups of people, most notably Northern Europeans, and people from these groups can continue to ingest milk without gastrointestinal difficulties. With the development of dairy farming, an adult with active lactase would have a selective advantage in being able to consume calories from the readily available milk. Indeed, estimates suggest that people with the mutation would produce almost 20% more fertile offspring. Because dairy farming appeared in northern Europe about 10,000 years ago, the evolutionary selective pressure on lactase persistence must have been substantial, attesting to the biochemical value of being able to use milk as an energy source into adulthood.
What happens to the lactose in the intestine of a lactase-deficient person? The lactose is a good energy source for microorganisms in the colon, and they ferment it to lactic acid while generating methane (CH4) and hydrogen gas (H2). The gas produced creates the uncomfortable feeling of gut distension and the annoying problem of flatulence. The lactate produced by the microorganisms is osmotically active and draws water into the intestine, as does any undigested lactose, resulting in diarrhea. If severe enough, the gas and diarrhea hinder the absorption of other nutrients such as fats and proteins. The simplest treatment is to avoid the consumption of products containing much lactose. Alternatively, the enzyme lactase can be ingested with milk products.
Galactose is highly toxic if the transferase is missing
 Less common than lactose intolerance are disorders that interfere with the metabolism of galactose. The disruption of galactose metabolism is referred to as galactosemia. The most common form, called classic galactosemia, is an inherited deficiency in galactose 1-phosphate uridyl transferase activity. Afflicted infants fail to thrive. They vomit or have diarrhea after consuming milk, and enlargement of the liver and jaundice are common, sometimes progressing to cirrhosis. Cataracts will form, and lethargy and retarded mental development also are common. The blood-galactose level is markedly elevated, and galactose is found in the urine. The absence of the transferase in red blood cells is a definitive diagnostic criterion.
Less common than lactose intolerance are disorders that interfere with the metabolism of galactose. The disruption of galactose metabolism is referred to as galactosemia. The most common form, called classic galactosemia, is an inherited deficiency in galactose 1-phosphate uridyl transferase activity. Afflicted infants fail to thrive. They vomit or have diarrhea after consuming milk, and enlargement of the liver and jaundice are common, sometimes progressing to cirrhosis. Cataracts will form, and lethargy and retarded mental development also are common. The blood-galactose level is markedly elevated, and galactose is found in the urine. The absence of the transferase in red blood cells is a definitive diagnostic criterion.
The most common treatment is to remove galactose (and lactose) from the diet. An enigma of galactosemia is that, although elimination of galactose from the diet prevents liver disease and cataract development, the majority of patients still suffer from central nervous system malfunction, most commonly a delayed acquisition of language skills. Female patients also display ovarian failure.
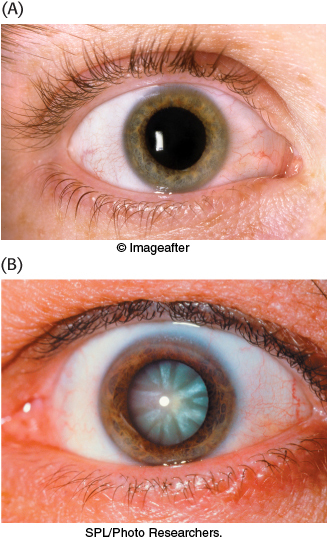
FIGURE 16.15Cataracts are evident as the clouding of the lens. (A) A healthy eye. (B) An eye with a cataract.
[(A) © Imageafter; (B) SPL/Photo Researchers.]
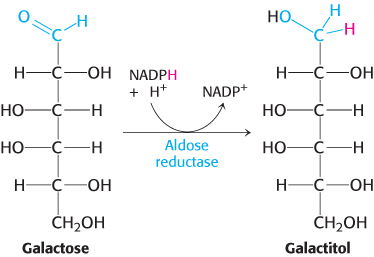
Cataract formation is better understood. A cataract is the clouding of the normally clear lens of the eye (Figure 16.15). If the transferase is not active in the lens of the eye, the presence of aldose reductase causes the accumulating galactose to be reduced to galactitol.
Galactitol is poorly metabolized and accumulates in the lens. Water will diffuse into the lens to maintain osmotic balance, triggering the formation of cataracts. In fact, there is a high incidence of cataract formation with age in populations that consume substantial amounts of milk into adulthood.




 The newly formed fructose 1,6-
The newly formed fructose 1,6-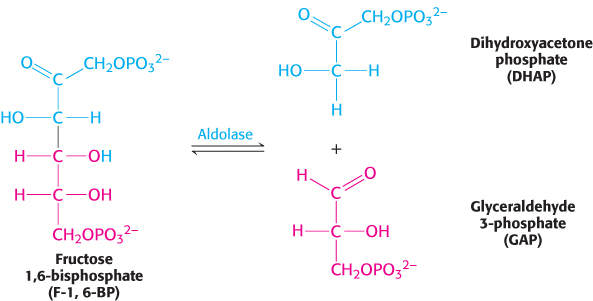

 FIGURE 16.4 Structure of triose phosphate isomerase. This enzyme consists of a central core of eight parallel β strands (orange) surrounded by eight α helices (blue). This structural motif, called an αβ barrel, is also found in the glycolytic enzymes aldolase, enolase, and pyruvate kinase. Notice that histidine 95 and glutamate 165, essential components of the active site of triose phosphate isomerase, are located in the barrel. A loop (red) closes off the active site on substrate binding.
FIGURE 16.4 Structure of triose phosphate isomerase. This enzyme consists of a central core of eight parallel β strands (orange) surrounded by eight α helices (blue). This structural motif, called an αβ barrel, is also found in the glycolytic enzymes aldolase, enolase, and pyruvate kinase. Notice that histidine 95 and glutamate 165, essential components of the active site of triose phosphate isomerase, are located in the barrel. A loop (red) closes off the active site on substrate binding.


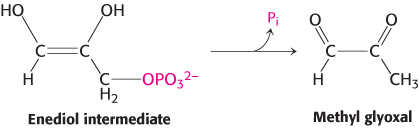
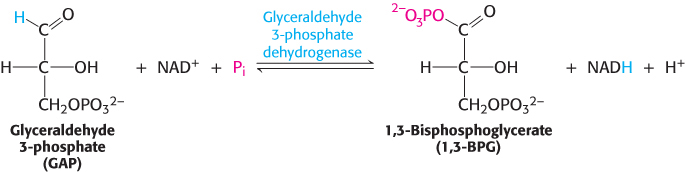




 FIGURE 16.7 Structure of glyceraldehyde 3-
FIGURE 16.7 Structure of glyceraldehyde 3-
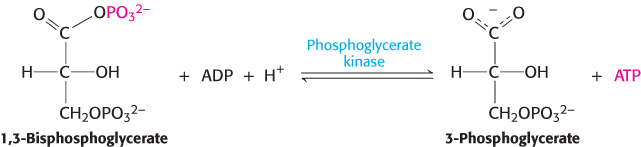
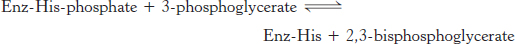
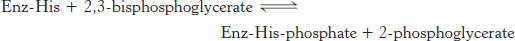


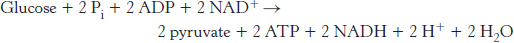


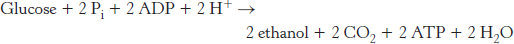


 The three dehydrogenases—
The three dehydrogenases—


 Fructose, a commonly used sweetener, is a component of sucrose and high fructose corn syrup (which contains approximately 55% fructose and 45% glucose). Epidemiological as well as clinical studies have linked excessive fructose consumption to fatty liver, insulin insensitivity, and obesity. These conditions may eventually result in type 2 diabetes (Chapter 27). Studies have shown that these disorders are not necessarily the result of simple excess energy consumption, but rather how fructose is processed by the liver. What aspects of liver fructose metabolism are the contributing factors then? Note that, as shown in Figure 16.14, the actions of fructokinase and triose kinase bypass the most important regulatory step in glycolysis, the phosphofructokinase-
Fructose, a commonly used sweetener, is a component of sucrose and high fructose corn syrup (which contains approximately 55% fructose and 45% glucose). Epidemiological as well as clinical studies have linked excessive fructose consumption to fatty liver, insulin insensitivity, and obesity. These conditions may eventually result in type 2 diabetes (Chapter 27). Studies have shown that these disorders are not necessarily the result of simple excess energy consumption, but rather how fructose is processed by the liver. What aspects of liver fructose metabolism are the contributing factors then? Note that, as shown in Figure 16.14, the actions of fructokinase and triose kinase bypass the most important regulatory step in glycolysis, the phosphofructokinase-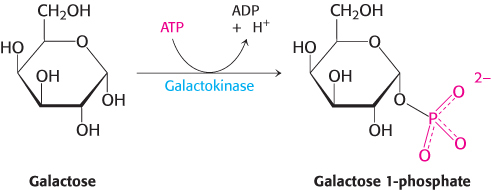
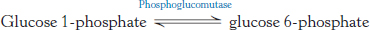
 Many adults are unable to metabolize the milk sugar lactose and experience gastrointestinal disturbances if they drink milk. Lactose intolerance, or hypolactasia, is most commonly caused by a deficiency of the enzyme lactase, which cleaves lactose into glucose and galactose.
Many adults are unable to metabolize the milk sugar lactose and experience gastrointestinal disturbances if they drink milk. Lactose intolerance, or hypolactasia, is most commonly caused by a deficiency of the enzyme lactase, which cleaves lactose into glucose and galactose.

 Less common than lactose intolerance are disorders that interfere with the metabolism of galactose. The disruption of galactose metabolism is referred to as galactosemia. The most common form, called classic galactosemia, is an inherited deficiency in galactose 1-
Less common than lactose intolerance are disorders that interfere with the metabolism of galactose. The disruption of galactose metabolism is referred to as galactosemia. The most common form, called classic galactosemia, is an inherited deficiency in galactose 1-