Signaling cascades lead to the mobilization of glycogen to produce glucose, an energy source for cyclists.
[(Left) Jonathan Devich/Epic Images.]
Glucose is an important fuel and, as we will see, a key precursor for the biosynthesis of many molecules. However, glucose cannot be stored, because high concentrations of glucose disrupt the osmotic balance of the cell, which would cause cell damage or death. How can adequate stores of glucose be maintained without damaging the cell? The solution to this problem is to store glucose as a nonosmotically active polymer called glycogen.
Glycogen is a readily mobilized storage form of glucose. It is a very large, branched polymer of glucose residues that can be broken down to yield glucose molecules when energy is needed (Figure 21.1). A glycogen molecule has approximately 12 layers of glucose molecules and can be as large as 40 nm, containing approximately 55,000 glucose residues. Most of the glucose residues in glycogen are linked by α-1,4-glycosidic bonds (Figure 21.2). Branches at about every 12 residues are created by α-1,6-glycosidic bonds. Recall that α-glycosidic linkages form open helical polymers, whereas β linkages produce nearly straight strands that form structural fibrils, as in cellulose (Figure 11.14).
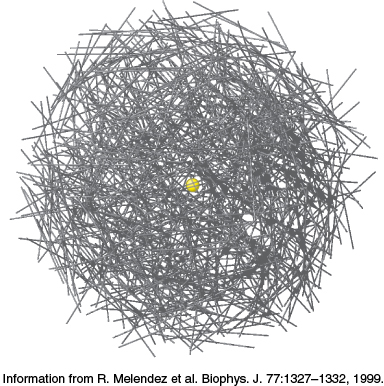
FIGURE 21.1Glycogen. At the core of the glycogen molecule is the protein glycogenin (yellow). Each line represents glucose molecules joined by an α-1,4-glycosidic linkage. The nonreducing ends of the glycogen molecule form the surface of the glycogen granule. Degradation takes place at this surface.
[Information from R. Melendez et al. Biophys. J. 77:1327–1332, 1999.]
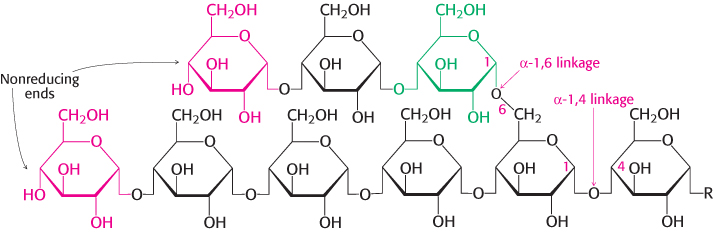
FIGURE 21.2Glycogen structure. In this structure of two outer branches of a glycogen molecule, the residues at the nonreducing ends are shown in red and the residue that starts a branch is shown in green. The rest of the glycogen molecule is represented by R.
Glycogen is not as reduced as fatty acids are and consequently are not as energy rich. Why isn’t all excess fuel stored as fatty acids rather than as glycogen? The controlled release of glucose from glycogen maintains blood-glucose concentration between meals. The circulating blood keeps the brain supplied with glucose, which is virtually the only fuel used by the brain, except during prolonged starvation (Chapter 27). Moreover, the readily mobilized glucose from glycogen is a good source of energy for sudden, strenuous activity. Unlike fatty acids, the released glucose can be metabolized in the absence of oxygen and can thus supply energy for anaerobic activity (Section 16.1).
Glycogen is present in bacteria, archae, and eukaryotes. Recall that plants store glucose as starch, a similar chemical. Thus, storing energy as glucose polymers is common to all forms of life. In humans, most tissues have some glycogen, although the two major sites of glycogen storage are the liver and skeletal muscle. The concentration of glycogen is higher in the liver than in muscle (10% versus 2% by weight), but more glycogen is stored in skeletal muscle overall because of muscle’s much greater mass. Glycogen is present in the cytoplasm, with the molecule appearing as granules (Figure 21.3). In the liver, glycogen synthesis and degradation are regulated to maintain blood-glucose levels as required to meet the needs of the organism as a whole. In contrast, in muscle, these processes are regulated to meet the energy needs of the muscle itself.
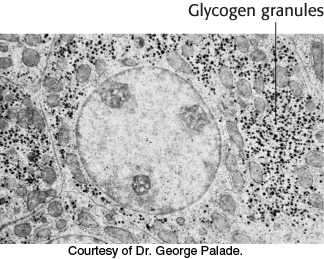
FIGURE 21.3Electron micrograph of a liver cell. The dark particles in the cytoplasm are glycogen granules.
[Courtesy of Dr. George Palade.]
Glycogen metabolism is the regulated release and storage of glucose
Glycogen degradation and synthesis are simple biochemical processes. Glycogen degradation consists of three steps: (1) the release of glucose 1-phosphate from glycogen, (2) the remodeling of the glycogen substrate to permit further degradation, and (3) the conversion of glucose 1-phosphate into glucose 6-phosphate for further metabolism. The glucose 6-phosphate derived from the breakdown of glycogen has three possible fates (Figure 21.4): (1) It can be metabolized by glycolysis, (2) it can be converted into free glucose for release into the bloodstream, and (3) it can be processed by the pentose phosphate pathway to yield NADPH and ribose derivatives. The conversion of glycogen into free glucose takes place mainly in the liver.
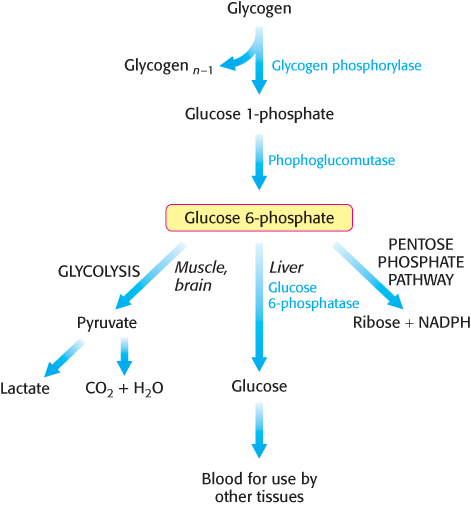
FIGURE 21.4Fates of glucose 6-phosphate. Glucose 6-phosphate derived from glycogen can (1) be used as a fuel for anaerobic or aerobic metabolism as in, for instance, muscle; (2) be converted into free glucose in the liver and subsequently released into the blood; (3) be processed by the pentose phosphate pathway to generate NADPH or ribose in a variety of tissues.
Glycogen synthesis, which takes place when glucose is abundant, requires an activated form of glucose, uridine diphosphate glucose (UDP-glucose), formed by the reaction of UTP and glucose 1-phosphate. As is the case for glycogen degradation, the glycogen molecule must be remodeled for continued synthesis.
The regulation of glycogen degradation and synthesis is complex and is facilitated by the fact that all of the enzymes involved in glycogen metabolism and its regulation are associated with the glycogen particle. Several enzymes taking part in glycogen metabolism allosterically respond to metabolites that signal the energy needs of the cell. Through these allosteric responses, enzyme activity is adjusted to meet the needs of the cell. In addition, hormones may initiate signal cascades that lead to the reversible phosphorylation of enzymes, which alters their catalytic rates. Regulation by hormones adjusts glycogen metabolism to meet the needs of the entire organism.




