21.4Glycogen Is Synthesized and Degraded by Different Pathways
Glycogen Is Synthesized and Degraded by Different Pathways
As with glycolysis and gluconeogenesis, biosynthetic and degradative pathways rarely operate by precisely the same reactions in the forward and reverse directions. Glycogen metabolism provided the first known example of this important principle. Separate pathways afford much greater flexibility, both in energetics and in control.
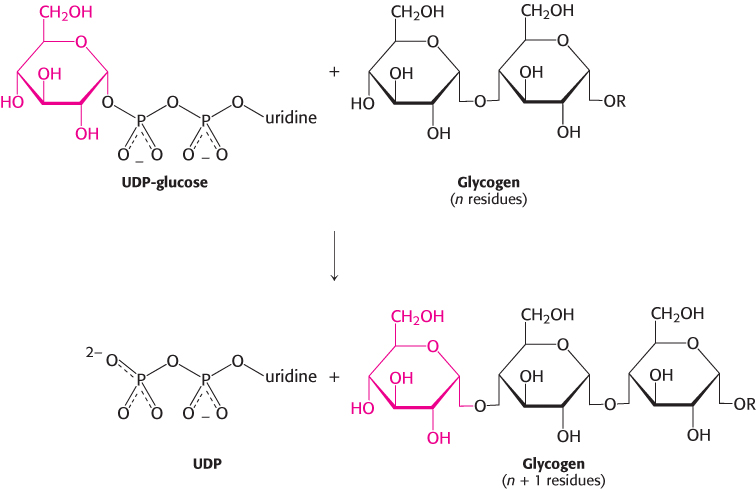
Glycogen is synthesized by a pathway that utilizes uridine diphosphate glucose (UDP-
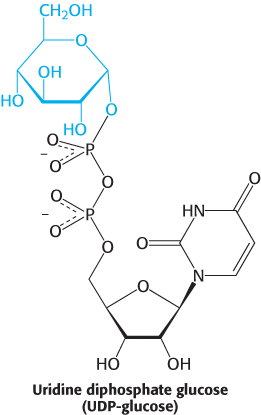
UDP-glucose is an activated form of glucose
UDP-
UDP-
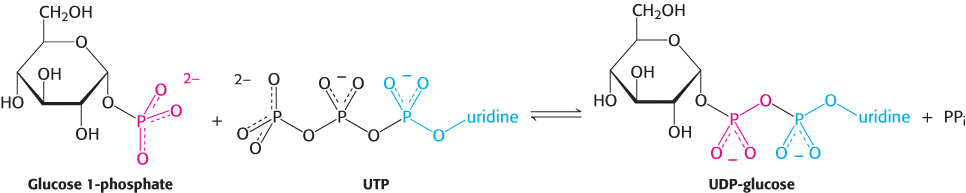
This reaction is readily reversible. However, pyrophosphate is rapidly hydrolyzed in vivo to orthophosphate by an inorganic pyrophosphatase. The essentially irreversible hydrolysis of pyrophosphate drives the synthesis of UDP-
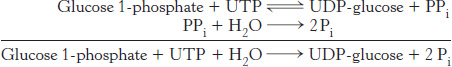
The synthesis of UDP-
Glycogen synthase catalyzes the transfer of glucose from UDP-glucose to a growing chain
New glucosyl units are added to the nonreducing terminal residues of glycogen. The activated glucosyl unit of UDP-
631
Glycogen synthase, a member of the large glycosyltransferase family (Section 11.3), can add glucosyl residues only to a polysaccharide chain already containing at least four residues. Thus, glycogen synthesis requires a primer. This priming function is carried out by glycogenin, a Mn2+-requiring glycosyltransferase composed of two identical 37-
 Despite no detectable sequence similarity, structural studies have revealed that glycogen synthase is homologous to glycogen phosphorylase. The binding site for UDP-
Despite no detectable sequence similarity, structural studies have revealed that glycogen synthase is homologous to glycogen phosphorylase. The binding site for UDP-
A branching enzyme forms α-1,6 linkages
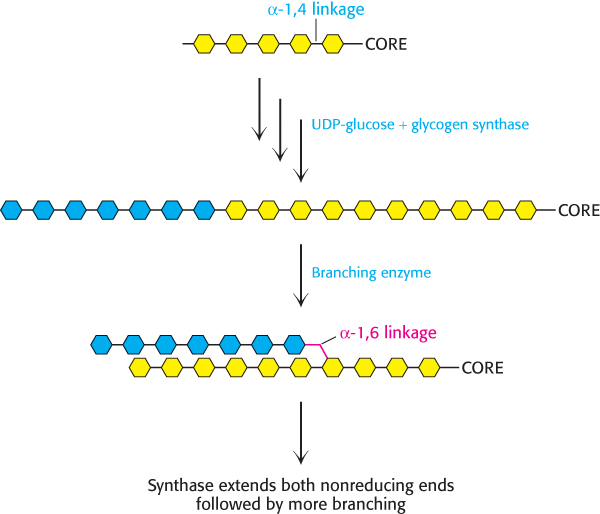
Glycogen synthase catalyzes only the synthesis of α-1,4 linkages. Another enzyme is required to form the α-1,6 linkages that make glycogen a branched polymer. Branching takes place after a number of glucosyl residues are joined in α-1,4 linkages by glycogen synthase (Figure 21.17). A branch is created by the breaking of an α-1,4 link and the formation of an α-1,6 link. A block of residues, typically 7 in number, is transferred to a more interior site. The branching enzyme that catalyzes this reaction requires that the block of 7 or so residues must include the nonreducing terminus, and must come from a chain at least 11 residues long. In addition, the new branch point must be at least 4 residues away from a preexisting one.
632
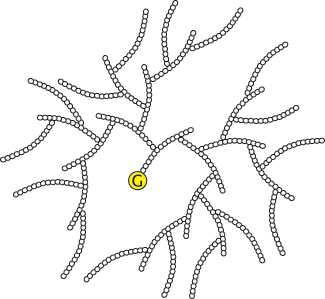
Branching is important because it increases the solubility of glycogen. Furthermore, branching creates a large number of terminal residues, the sites of action of glycogen phosphorylase and synthase (Figure 21.18). Thus, branching increases the rate of glycogen synthesis and degradation.
Glycogen synthase is the key regulatory enzyme in glycogen synthesis
Glycogen synthase, like phosphorylase, exists in two forms: an active nonphosphorylated a form and a usually inactive phosphorylated b form. Again, like the phosphorylase, the interconversion of the two forms is regulated by covalent modification. However, the key means of regulating glycogen synthase is by allosteric regulation of the phosphorylated form of the enzyme, glycogen synthase b. Glucose 6-
The covalent modification of glycogen synthase appears to play more of a fine-
Glycogen is an efficient storage form of glucose
What is the cost of converting glucose 6-
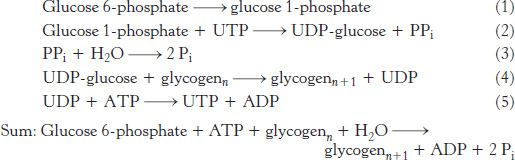
Thus, 1 molecule of ATP is hydrolyzed to incorporate glucose 6-