21.2Phosphorylase Is Regulated by Allosteric Interactions and Reversible Phosphorylation
Phosphorylase Is Regulated by Allosteric Interactions and Reversible Phosphorylation
Glycogen degradation is precisely controlled by multiple interlocking mechanisms. The focus of this control is the enzyme glycogen phosphorylase. Phosphorylase is regulated by several allosteric effectors that signal the energy state of the cell as well as by reversible phosphorylation, which is responsive to hormones such as insulin, epinephrine, and glucagon. We will examine the differences in the control of two isozymic forms of glycogen phosphorylase: one specific to liver and one specific to skeletal muscle. These differences are due to the fact that the liver maintains glucose homeostasis of the organism as a whole, whereas the muscle uses glucose to produce energy for itself.
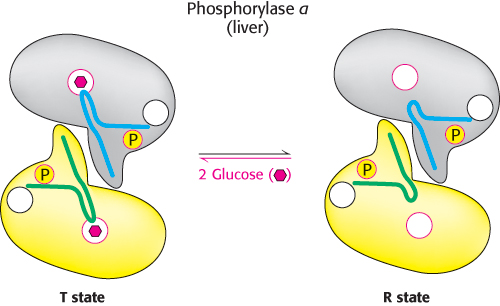
Liver phosphorylase produces glucose for use by other tissues
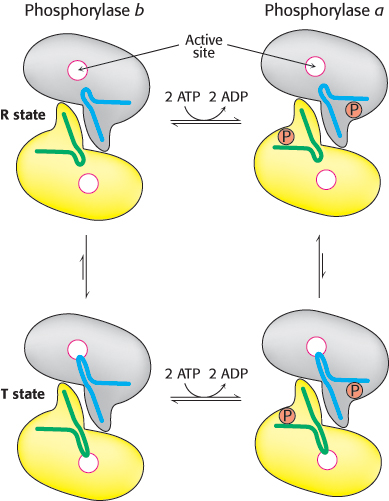
The dimeric phosphorylase exists in two interconvertible forms: a usually active phosphorylase a and a usually inactive phosphorylase b (Figure 21.10). Each of these two forms exists in equilibrium between an active relaxed (R) state and a much less active tense (T) state, but the equilibrium for phosphorylase a favors the active R state, whereas the equilibrium for phosphorylase b favors the less-
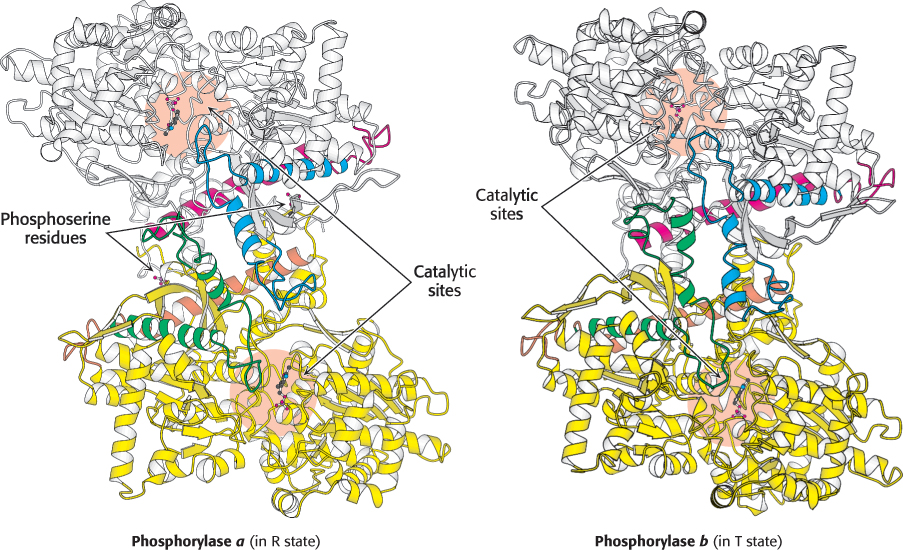
 FIGURE 21.10 Structures of phosphorylase a and phosphorylase b. Phosphorylase a is phosphorylated on serine 14 of each subunit. This modification favors the structure of the more-
FIGURE 21.10 Structures of phosphorylase a and phosphorylase b. Phosphorylase a is phosphorylated on serine 14 of each subunit. This modification favors the structure of the more-624
625
Muscle phosphorylase is regulated by the intracellular energy charge
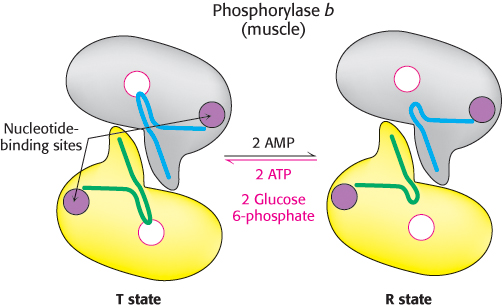
In contrast to the liver isozyme, the default state of muscle phosphorylase is the b form, owing to the fact that, for muscle, phosphorylase must be active primarily during muscle contraction. Muscle phosphorylase b is activated by the presence of high concentrations of AMP, which binds to a nucleotide-
Unlike the enzyme in muscle, the liver phosphorylase is insensitive to regulation by AMP because the liver does not undergo the dramatic changes in energy charge seen in a contracting muscle. We see here a clear example of the use of isozymes to establish the tissue-
Biochemical characteristics of muscle fiber types differ
Not only do the biochemical needs of liver and muscle differ with respect to glycogen metabolism, but the biochemical needs of different muscle fiber types also vary. Skeletal muscle consists of three different fiber types: type I, or slow-
|
Characteristic |
Type I |
Type IIa |
Type IIb |
|---|---|---|---|
|
Fatigue resistance |
High |
Intermediate |
Low |
|
Mitochondrial density |
High |
Intermediate |
Low |
|
Metabolic type |
Oxidative |
Oxidative/glycolytic |
Glycolytic |
|
Myoglobin content |
High |
Intermediate |
Low |
|
Glycogen content |
Low |
Intermediate |
High |
|
Triacylglycerol content |
High |
Intermediate |
Low |
|
Glycogen phosphorylase activity |
Low |
Intermediate |
High |
|
Phosphofructokinase activity |
Low |
Intermediate |
High |
|
Citrate synthase activity |
High |
Intermediate |
Low |
626
Phosphorylation promotes the conversion of phosphorylase b to phosphorylase a
In both liver and muscle, phosphorylase b is converted into phosphorylase a by the phosphorylation of a single serine residue (serine 14) in each subunit. This conversion is initiated by hormones. Low blood levels of glucose lead to the secretion of the hormone glucagon. Fear or the excitement of exercise will cause levels of the hormone epinephrine to increase. The rise in glucagon and epinephrine levels results in phosphorylation of the enzyme to the phosphorylase a form in liver and muscle respectively. The regulatory enzyme phosphorylase kinase catalyzes this covalent modification.
Comparison of the structures of phosphorylase a in the R state and phosphorylase b in the T state reveals that subtle structural changes at the subunit interfaces are transmitted to the active sites (Figure 21.10). The transition from the T state (the prevalent state of phosphorylase b) to the R state (the prevalent state of phosphorylase a) entails a 10-
Phosphorylase kinase is activated by phosphorylation and calcium ions
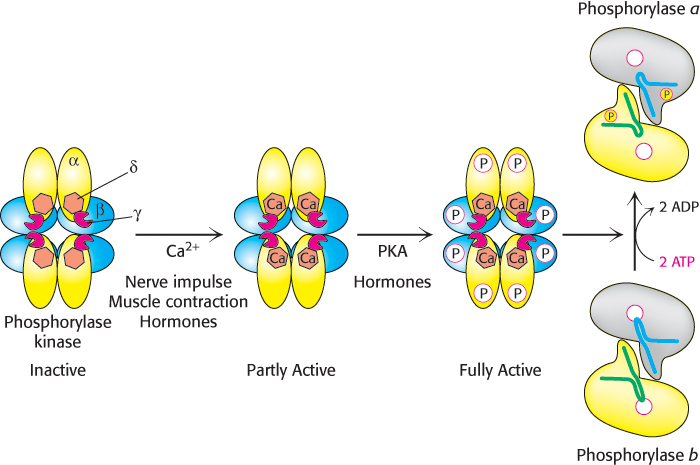
Phosphorylase kinase activates phosphorylase b by attaching a phosphoryl group. The subunit composition of phosphorylase kinase in skeletal muscle is (αβγδ)4, and the mass of this very large protein is 1300 kDa. The enzyme consists of two (αβγδ)2 lobes that are joined by a β4 bridge that is the core of the enzyme and serves as a scaffold for the remaining subunits. The γ subunit contains the active site, while all of the remaining subunits (≈90% by mass) play regulatory roles. The δ subunit is the calcium binding protein calmodulin, a calcium sensor that stimulates many enzymes in eukaryotes (Section 14.1). The α and β subunits are targets of protein kinase A. The β subunit is phosphorylated first, followed by the phosphorylation of the α subunit.
Activation of phosphorylase kinase is initiated when Ca2+ binds to the δ subunit. This mode of activation of the kinase is especially noteworthy in muscle, where contraction is triggered by the release of Ca2+ from the sarcoplasmic reticulum (Figure 21.14). Maximal activation is achieved with the phosphorylation of the β and α subunits of the Ca2+-bound kinase. The stimulation of phosphorylase kinase is one step in a signal-
627