9.4Myosins Harness Changes in Enzyme Conformation to Couple ATP Hydrolysis to Mechanical Work
Myosins Harness Changes in Enzyme Conformation to Couple ATP Hydrolysis to Mechanical Work
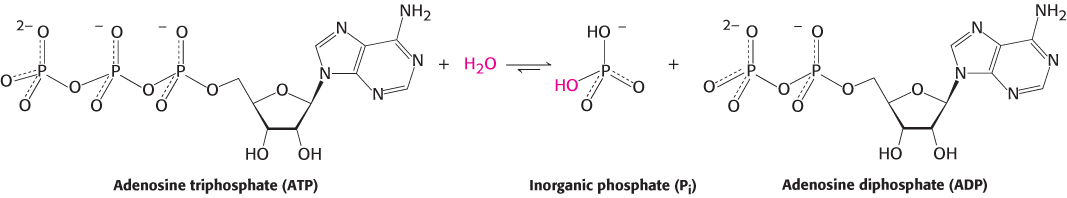
The final enzymes that we will consider are the myosins. These enzymes catalyze the hydrolysis of adenosine triphosphate (ATP) to form adenosine diphosphate (ADP) and inorganic phosphate (Pi) and use the energy associated with this thermodynamically favorable reaction to drive the motion of molecules within cells.
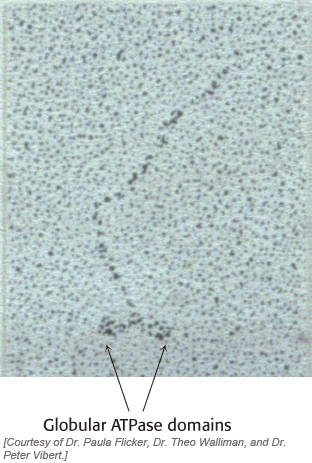
For example, when we lift a book, the energy required comes from ATP hydrolysis catalyzed by myosin in our muscles. Myosins are found in all eukaryotes and the human genome encodes more than 40 different myosins. Myosins generally have elongated structures with globular domains that actually carry out ATP hydrolysis (Figure 9.42). In this chapter, we will focus on the globular ATPase domains, particularly the strategies that allow myosins to hydrolyze ATP in a controlled manner and to use the free energy associated with this reaction to promote substantial conformational changes within the myosin molecule. These conformational changes are amplified by other structures in the elongated myosin molecules to transport proteins or other cargo substantial distances within cells. In Chapter 35, we will examine the action of myosins and other molecular-
276
As will be discussed in Chapter 15, ATP is used as the major currency of energy inside cells. Many enzymes use ATP hydrolysis to drive other reactions and processes. In almost all cases, an enzyme that hydrolyzed ATP without any such coupled processes would simply drain the energy reserves of a cell without benefit.
ATP hydrolysis proceeds by the attack of water on the gamma phosphoryl group
In our examination of the mechanism of restriction enzymes, we learned that an activated water molecule performs a nucleophilic attack on phosphorus to cleave the phosphodiester backbone of DNA. The cleavage of ATP by myosins follows an analogous mechanism. To understand the myosin mechanism in more detail, we must first examine the structure of the myosin ATPase domain.
The structures of the ATPase domains of several different myosins have been examined. One such domain, that from the soil-
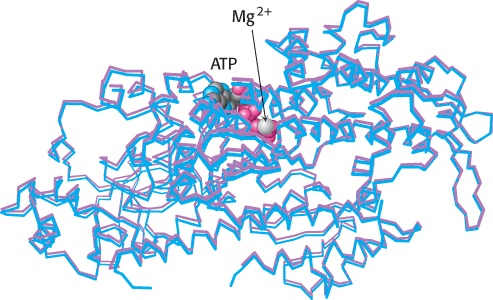
 FIGURE 9.43 Myosin–
FIGURE 9.43 Myosin–Kinetic studies of myosins, as well as many other enzymes having ATP or other nucleoside triphosphates as a substrate, reveal that these enzymes are essentially inactive in the absence of divalent metal ions such as magnesium (Mg2+) or manganese (Mn2+) but acquire activity on the addition of these ions. In contrast with the enzymes discussed so far, the metal is not a component of the active site. Rather, nucleotides such as ATP bind these ions, and it is the metal ion–
The nucleophilic attack by a water molecule on the γ-phosphoryl group requires some mechanism to activate the water, such as a basic residue or a bound metal ion. Examination of the myosin–
277
Formation of the transition state for ATP hydrolysis is associated with a substantial conformational change
The catalytically competent conformation of the myosin ATPase domain must bind and stabilize the transition state of the reaction. In analogy with restriction enzymes, we expect that ATP hydrolysis includes a pentacoordinate transition state.
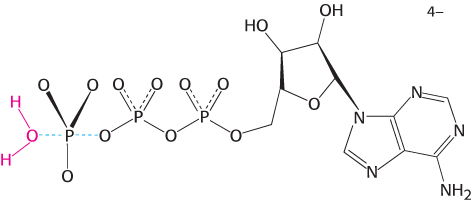
Such pentacoordinate structures based on phosphorus are too unstable to be readily observed. However, transition-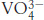 . The result is the formation of a complex that closely matches the expected transition-
. The result is the formation of a complex that closely matches the expected transition-
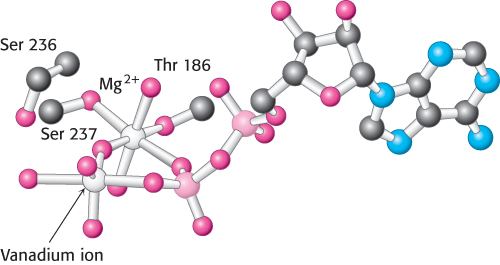
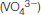 in the presence of magnesium. Notice that the vanadium ion is coordinated to five oxygen atoms including one from ADP. The positions of two residues that bind magnesium as well as Ser 236, a residue that appears to play a direct role in catalysis, are shown.
in the presence of magnesium. Notice that the vanadium ion is coordinated to five oxygen atoms including one from ADP. The positions of two residues that bind magnesium as well as Ser 236, a residue that appears to play a direct role in catalysis, are shown.
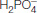 product.
product.
278
Comparison of the overall structures of the myosin ATPase domain complexed with ATP and with the ADP–
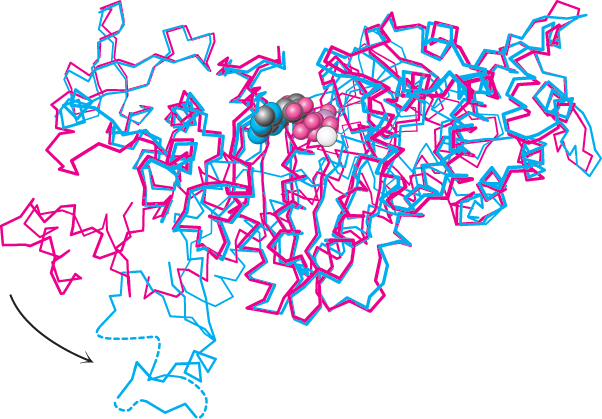
 FIGURE 9.46 Myosin conformational changes. A comparison of the overall structures of the myosin ATPase domain with ATP bound (shown in red) and that with the transition-
FIGURE 9.46 Myosin conformational changes. A comparison of the overall structures of the myosin ATPase domain with ATP bound (shown in red) and that with the transition-A region comprising approximately 60 amino acids at the carboxyl-
The altered conformation of myosin persists for a substantial period of time
Myosins are slow enzymes, typically turning over approximately once per second. What steps limit the rate of turnover? In an experiment that was particularly revealing, the hydrolysis of ATP was catalyzed by the myosin ATPase domain from mammalian muscle. The reaction took place in water labeled with 18O to track the incorporation of solvent oxygen into the reaction products. The fraction of oxygen in the phosphate product was analyzed. In the simplest case, the phosphate would be expected to contain one oxygen atom derived from water and three initially present in the terminal phosphoryl group of ATP.
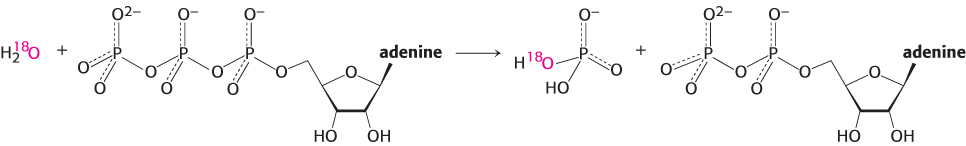
279
Instead, between two and three of the oxygen atoms in the phosphate were found, on average, to be derived from water. These observations indicate that the ATP hydrolysis reaction within the enzyme active site is reversible. Each molecule of ATP is cleaved to ADP and Pi and then re-
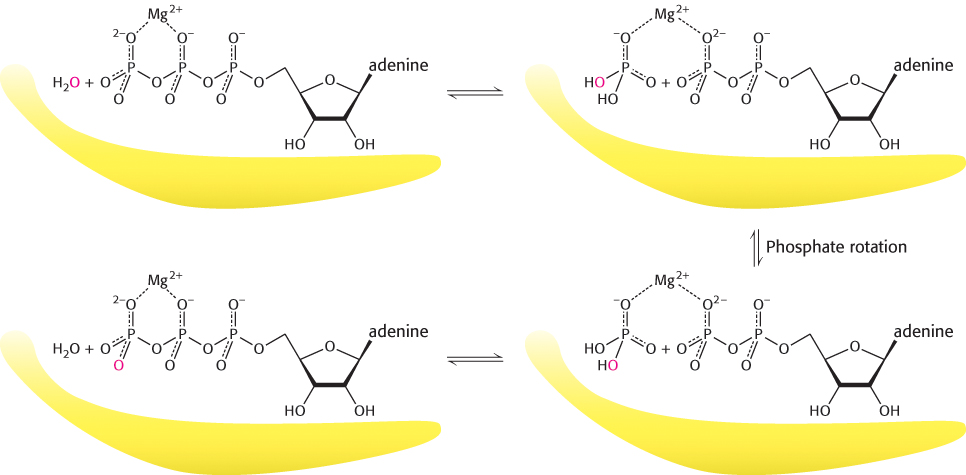
These observations reveal that the hydrolysis of ATP to ADP and Pi is not the rate-
Scientists can watch single molecules of myosin move
Myosin molecules function to use the free energy of hydrolysis of ATP to drive macroscopic motion. Myosin molecules move along a filamentous protein termed actin, as we will discuss in more detail in Chapter 35. Using a variety of physical methods, scientists have been able to watch single myosin in action. For example, a myosin family member termed myosin V can be labelled with fluorescent tags so that it can be localized when fixed on a surface with a precision of less than 15 Å. When this myosin is placed on a surface coated with actin filaments, each molecule remains in a fixed position. However, when ATP is added, each molecule moves along the surface. Tracking individual molecules reveals that each moves in steps of approximately 74 nm as shown in Figure 9.48. The observation of steps of a fixed size as well as the determination of this step size helps reveal details of the mechanism of action of these tiny molecular motors.
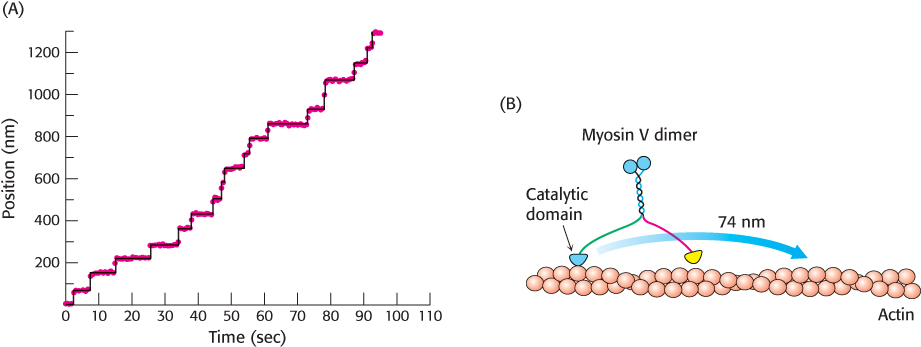
280
Myosins are a family of enzymes containing P-loop structures
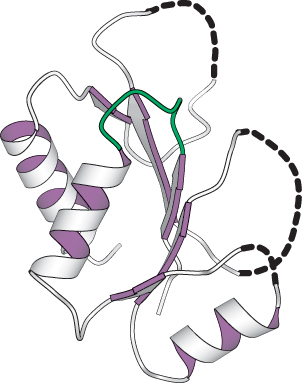
 FIGURE 9.49 The core domain of NMP kinases. Notice the P-
FIGURE 9.49 The core domain of NMP kinases. Notice the P- X-
X-
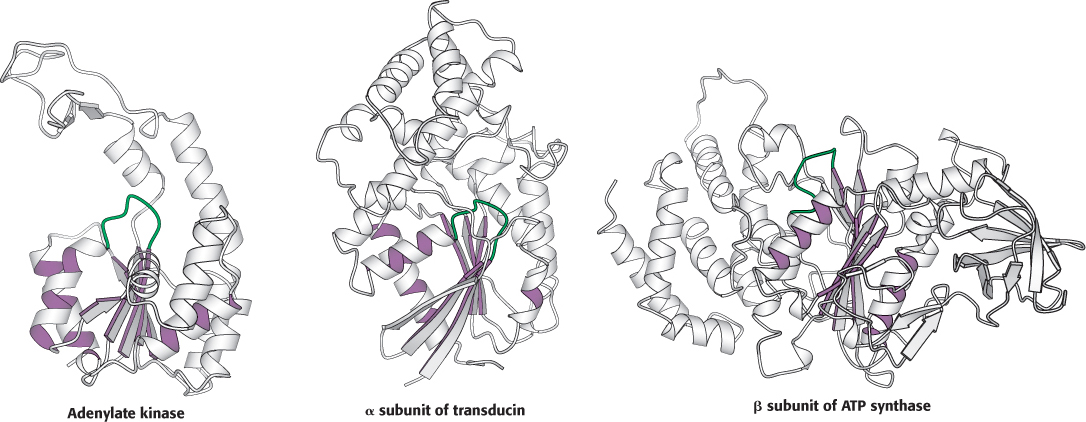
 FIGURE 9.50 Three proteins containing P-
FIGURE 9.50 Three proteins containing P-281