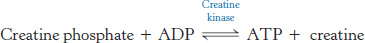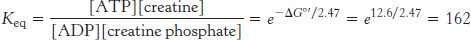15.2
ATP Is the Universal Currency of Free Energy in Biological Systems
Just as commerce is facilitated by the use of a common currency, the commerce of the cell—metabolism—is facilitated by the use of a common energy currency, adenosine triphosphate (ATP). Part of the free energy derived from the oxidation of foodstuffs and from light is transformed into this highly accessible molecule, which acts as the free-energy donor in most energy-requiring processes such as motion, active transport, and biosynthesis. Indeed, most of catabolism consists of reactions that extract energy from fuels such as carbohydrates and fats and convert it into ATP.
ATP hydrolysis is exergonic
ATP is a nucleotide consisting of adenine, a ribose, and a triphosphate unit (Figure 15.3). The active form of ATP is usually a complex of ATP with Mg2+ or Mn2+. In considering the role of ATP as an energy carrier, we can focus on its triphosphate moiety. ATP is an energy-rich molecule because its triphosphate unit contains two phosphoanhydride bonds. A large amount of free energy is liberated when ATP is hydrolyzed to adenosine diphosphate (ADP) and orthophosphate (Pi) or when ATP is hydrolyzed to adenosine monophosphate (AMP) and pyrophosphate (PPi).
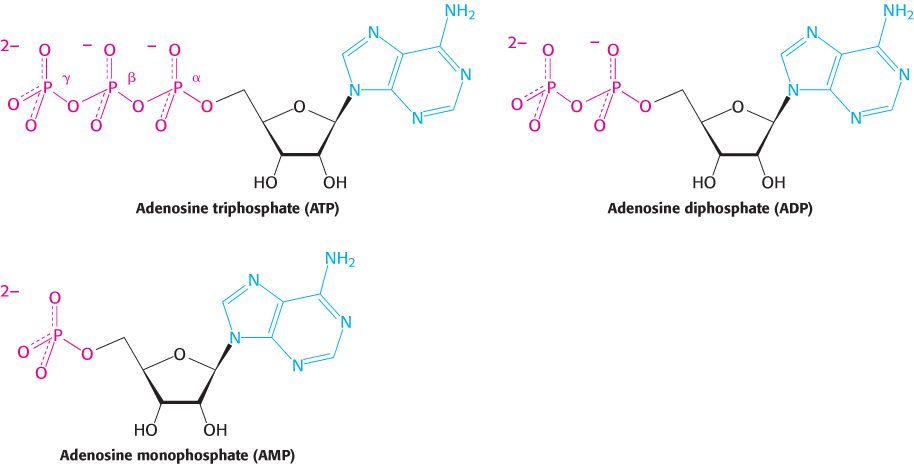
FIGURE 15.3Structures of ATP, ADP, and AMP. These adenylates consist of adenine (blue), a ribose (black), and a tri-, di-, or monophosphate unit (red). The innermost phosphorus atom of ATP is designated Pα, the middle one Pβ, and the outermost one Pγ.
The precise ΔG for these reactions depends on the ionic strength of the medium and on the concentrations of Mg2+ and other metal ions (problems 23 and 34). Under typical cellular concentrations, the ΔG for these hydrolyses is approximately −50 kJ mol−1 (−12 kcal mol−1).
The free energy liberated in the hydrolysis of ATP is harnessed to drive reactions that require an input of free energy, such as muscle contraction. In turn, ATP is formed from ADP and Pi when fuel molecules are oxidized in chemotrophs or when light is trapped by phototrophs. This ATP–ADP cycle is the fundamental mode of energy exchange in biological systems.
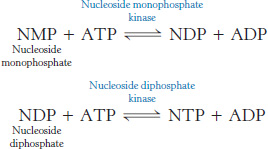
Some biosynthetic reactions are driven by the hydrolysis of other nucleoside triphosphates—namely, guanosine triphosphate (GTP), uridine triphosphate (UTP), and cytidine triphosphate (CTP). The diphosphate forms of these nucleotides are denoted by GDP, UDP, and CDP, and the monophosphate forms are denoted by GMP, UMP, and CMP. Enzymes catalyze the transfer of the terminal phosphoryl group from one nucleotide to another. The phosphorylation of nucleoside monophosphates is catalyzed by a family of nucleoside monophosphate kinases, as discussed in Section 9.4. The phosphorylation of nucleoside diphosphates is catalyzed by nucleoside diphosphate kinase, an enzyme with broad specificity.
It is intriguing to note that although all of the nucleotide triphosphates are energetically equivalent, ATP is nonetheless the primary cellular energy carrier. In addition, two important electron carriers, NAD+ and FAD, as well the acyl group carrier, coenzyme A, are derivatives of ATP. The role of ATP in energy metabolism is paramount.
ATP hydrolysis drives metabolism by shifting the equilibrium of coupled reactions
An otherwise unfavorable reaction can be made possible by coupling to ATP hydrolysis. Consider a reaction that is thermodynamically unfavorable without an input of free energy, a situation common to most biosynthetic reactions. Suppose that the standard free energy of the conversion of compound A into compound B is +16.7 kJ mol−1 (+4.0 kcal mol−1):
The equilibrium constant 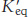 of this reaction at 25° C is related to ΔG°′ (in units of kilojoules per mole) by
of this reaction at 25° C is related to ΔG°′ (in units of kilojoules per mole) by
Thus, net conversion of A into B cannot take place when the molar ratio of B to A is equal to or greater than 1.15 × 10−3. However, A can be converted into B under these conditions if the reaction is coupled to the hydrolysis of ATP. Under standard conditions, the ΔG°′ of hydrolysis is approximately −30.5 kJ mol−1 (−7.3 kcal mol−1). The new overall reaction is
Its free-energy change of −13.8 kJ mol−1 (−3.3 kcal mol−1) is the sum of the value of ΔG°′ for the conversion of A into B [+16.7 kJ mol−1 (+4.0 kcal mol−1)] and the value of ΔG°′ for the hydrolysis of ATP [− 30.5 kJ mol−1 (− 7.3 kcal mol−1)]. At pH 7, the equilibrium constant of this coupled reaction is
At equilibrium, the ratio of [B] to [A] is given by
which means that the hydrolysis of ATP enables A to be converted into B until the [B]/[A] ratio reaches a value of 2.67 × 102. This equilibrium ratio is strikingly different from the value of 1.15 × 10−3 for the reaction A→B in the absence of ATP hydrolysis. In other words, coupling the hydrolysis of ATP with the conversion of A into B under standard conditions has changed the equilibrium ratio of B to A by a factor of about 105. If we were to use the ΔG of hydrolysis of ATP under cellular conditions [−50.2 kJ mol−1 (−12 kcal mol−1)] in our calculations instead of ΔG°′, the change in the equilibrium ratio would be even more dramatic, on the order of 108.
We see here the thermodynamic essence of ATP’s action as an energy-coupling agent. Cells maintain ATP levels by using oxidizable substrates or light as sources of free energy for synthesizing the molecule. In the cell, the hydrolysis of an ATP molecule in a coupled reaction then changes the equilibrium ratio of products to reactants by a very large factor, of the order of 108. More generally, the hydrolysis of n ATP molecules changes the equilibrium ratio of a coupled reaction (or sequence of reactions) by a factor of 108n. For example, the hydrolysis of three ATP molecules in a coupled reaction changes the equilibrium ratio by a factor of 1024. Thus, a thermodynamically unfavorable reaction sequence can be converted into a favorable one by coupling it to the hydrolysis of a sufficient number of ATP molecules in a new reaction. It should also be emphasized that A and B in the preceding coupled reaction may be interpreted very generally, not only as different chemical species. For example, A and B may represent activated and unactivated conformations of a protein that is activated by phosphorylation with ATP. Through such changes in protein conformation, molecular motors such as myosin, kinesin, and dynein convert the chemical energy of ATP into mechanical energy (Chapter 34). Indeed, this conversion is the basis of muscle contraction.
Alternatively, A and B may refer to the concentrations of an ion or molecule on the outside and inside of a cell, as in the active transport of a nutrient. The active transport of Na+ and K+ across membranes is driven by the phosphorylation of the sodium–potassium pump by ATP and its subsequent dephosphorylation (Section 13.2).
The high phosphoryl potential of ATP results from structural differences between ATP and its hydrolysis products
What makes ATP an efficient phosphoryl-group donor? Let us compare the standard free energy of hydrolysis of ATP with that of a phosphate ester, such as glycerol 3-phosphate:
The magnitude of ΔG°′ for the hydrolysis of glycerol 3-phosphate is much smaller than that of ATP, which means that ATP has a stronger tendency to transfer its terminal phosphoryl group to water than does glycerol 3-phosphate. In other words, ATP has a higher phosphoryl-transfer potential (phosphoryl-group-transfer potential) than does glycerol 3-phosphate.
The high phosphoryl-transfer potential of ATP can be explained by features of the ATP structure. Because ΔG°′ depends on the difference in free energies of the products and reactants, we need to examine the structures of both ATP and its hydrolysis products, ADP and Pi, to answer this question. Four factors are important: resonance stabilization, electrostatic repulsion, increase in entropy, and stabilization due to hydration.
Resonance Stabilization. Orthophosphate (Pi), one of the products of ATP hydrolysis, has greater resonance stabilization than do any of the phosphoryl groups of ATP. Orthophosphate has a number of resonance forms of similar energy (Figure 15.4), whereas the γ phosphoryl group of ATP has a smaller number. Forms like that shown in Figure 15.5 are unfavorable because a positively charged oxygen atom is adjacent to a positively charged phosphorus atom, an electrostatically unfavorable juxtaposition.
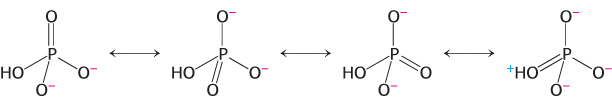
FIGURE 15.4Resonance structures of orthophosphate.
FIGURE 15.5Improbable resonance structure. The structure contributes little to the terminal part of ATP, because two positive charges are placed adjacent to each other.
Electrostatic Repulsion. At pH 7, the triphosphate unit of ATP carries about four negative charges. These charges repel one another because they are in close proximity. The repulsion between them is reduced when ATP is hydrolyzed.
Increase in Entropy. The entropy of the products of ATP hydrolysis is greater, in that there are now two molecules instead of a single ATP molecule. We disregard the molecule of water used to hydrolyze the ATP; given the high concentration (55.5 M), there is effectively no change in the concentration of water during the reaction.
Stabilization Due to Hydration. Water binds to ADP and Pi, stabilizing these molecules, and rendering the reverse reaction, the synthesis of ATP, more unfavorable.
ATP is often called a high-energy phosphate compound, and its phosphoanhydride bonds are referred to as high-energy bonds. Indeed, a “squiggle” (∼ P) is often used to indicate such a bond. Nonetheless, there is nothing special about the bonds themselves. They are high-energy bonds in the sense that much free energy is released when they are hydrolyzed, for the reasons listed above.
Phosphoryl-transfer potential is an important form of cellular energy transformation
The standard free energies of hydrolysis provide a convenient means of comparing the phosphoryl-transfer potential of phosphorylated compounds. Such comparisons reveal that ATP is not the only compound with a high phosphoryl-transfer potential. In fact, some compounds in biological systems have a higher phosphoryl-transfer potential than that of ATP. These compounds include phosphoenolpyruvate (PEP), 1,3-bisphosphoglycerate (1,3-BPG), and creatine phosphate (Figure 15.6). Thus, PEP can transfer its phosphoryl group to ADP to form ATP. Indeed, this transfer is one of the ways in which ATP is generated in the breakdown of sugars (Chapter 16). It is significant that ATP has a phosphoryl-transfer potential that is intermediate among the biologically important phosphorylated molecules (Table 15.1). This intermediate position enables ATP to function efficiently as a carrier of phosphoryl groups.
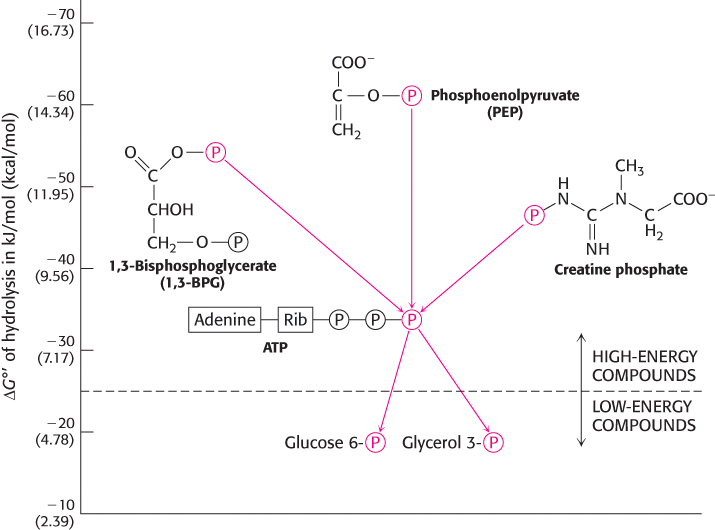
FIGURE 15.6Compounds with high phosphoryl-transfer potential. The role of ATP as the cellular energy currency is illustrated by its relation to other phosphorylated compounds. ATP has a phosphoryl-transfer potential that is intermediate among the biologically important phosphorylated molecules. High-phosphoryl-transfer-potential compounds (1,3-BPG, PEP, and creatine phosphate) derived from the metabolism of fuel molecules are used to power ATP synthesis. In turn, ATP donates a phosphoryl group to other biomolecules to facilitate their metabolism.
[Data from D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, 5th ed. (W. H. Freeman and Company, 2009), Fig. 13-19.]
TABLE 15.1 Standard free energies of hydrolysis of some phosphorylated compounds
The amount of ATP in muscle suffices to sustain contractile activity for less than a second. Creatine phosphate in vertebrate muscle serves as a reservoir of high-potential phosphoryl groups that can be readily transferred to ADP. Indeed, we use creatine phosphate to regenerate ATP from ADP every time that we exercise strenuously. This reaction is catalyzed by creatine kinase.
At pH 7, the standard free energy of hydrolysis of creatine phosphate is −43.1 kJ mol−1 (−10.3 kcal mol−1), compared with −30.5 kJ mol−1 (−7.3 kcal mol−1) for ATP. Hence, the standard free-energy change in forming ATP from creatine phosphate is −12.6 kJ mol−1 (−3.0 kcal mol−1), which corresponds to an equilibrium constant of 162.
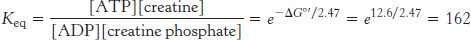
In resting muscle, typical concentrations of these metabolites are [ATP] = 4 mM, [ADP] = 0.013 mM, [creatine phosphate] = 25 mM, and [creatine] = 13 mM. Because of its abundance and high phosphoryl-transfer potential relative to that of ATP, creatine phosphate is a highly effective phosphoryl buffer. Indeed, creatine phosphate is the major source of phosphoryl groups for ATP regeneration for a runner during the first 4 seconds of a 100-meter sprint. The fact that creatine phosphate can replenish ATP pools is the basis of the use of creatine as a dietary supplement by athletes in sports requiring short bursts of intense activity. After the creatine phosphate pool is depleted, ATP must be generated through metabolism (Figure 15.7).
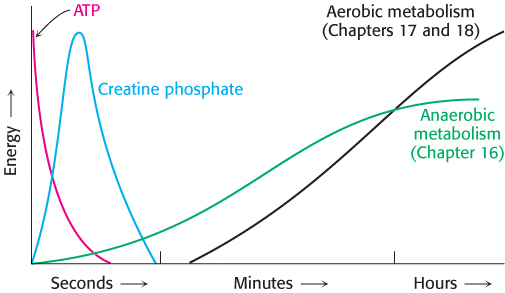
FIGURE 15.7Sources of ATP during exercise. In the initial seconds, exercise is powered by existing high-phosphoryl-transfer compounds (ATP and creatine phosphate). Subsequently, the ATP must be regenerated by metabolic pathways.
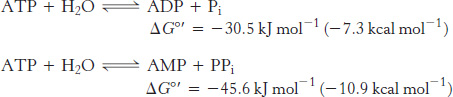


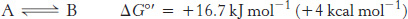
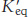 of this reaction at 25° C is related to ΔG°′ (in units of kilojoules per mole) by
of this reaction at 25° C is related to ΔG°′ (in units of kilojoules per mole) by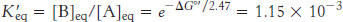
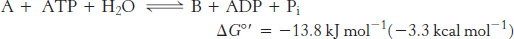
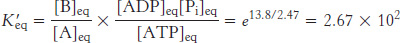
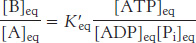
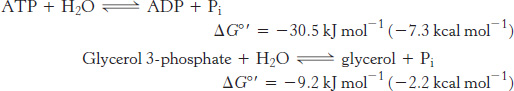
 FIGURE 15.4Resonance structures of orthophosphate.
FIGURE 15.4Resonance structures of orthophosphate. FIGURE 15.5Improbable resonance structure. The structure contributes little to the terminal part of ATP, because two positive charges are placed adjacent to each other.
FIGURE 15.5Improbable resonance structure. The structure contributes little to the terminal part of ATP, because two positive charges are placed adjacent to each other.