Glycolysis and Gluconeogenesis
CHAPTER
16
449
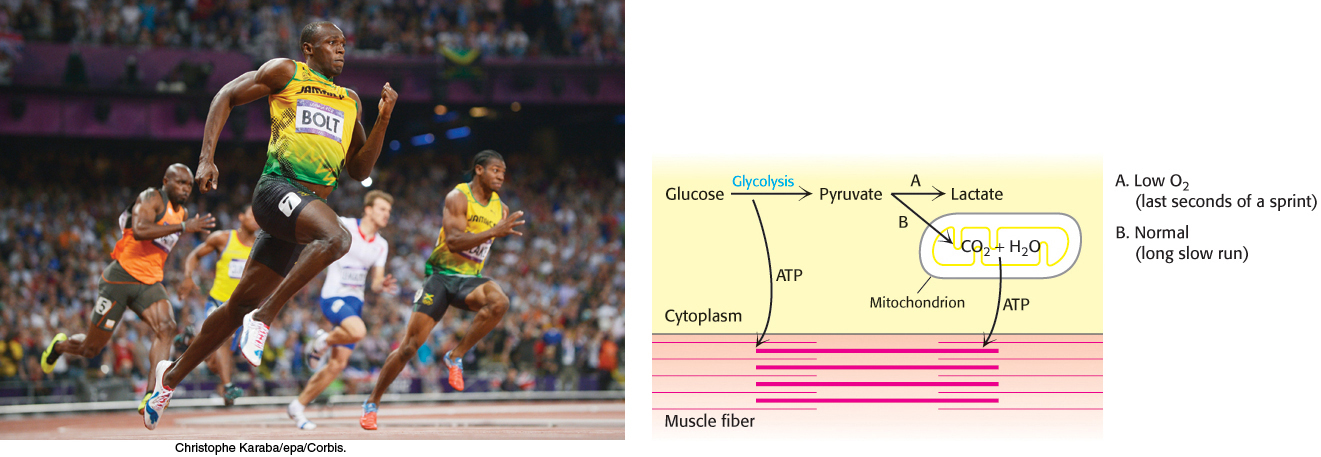
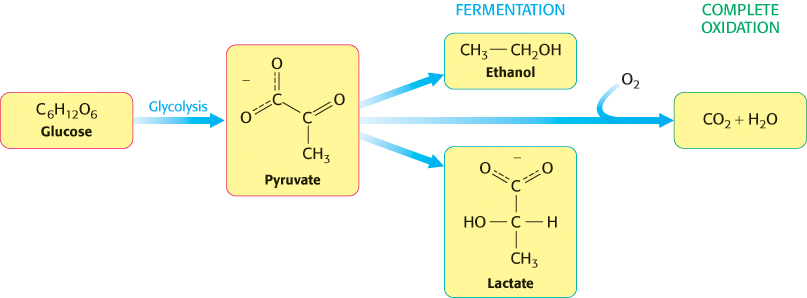
The first metabolic pathway that we encounter is glycolysis, an ancient pathway employed by a host of organisms. Glycolysis is the sequence of reactions that metabolizes one molecule of glucose to two molecules of pyruvate with the concomitant net production of two molecules of ATP. This process is anaerobic (i.e., it does not require O2) because it evolved before substantial amounts of oxygen accumulated in the atmosphere. Pyruvate can be further processed anaerobically to lactate (lactic acid fermentation) or ethanol (alcoholic fermentation). Under aerobic conditions, pyruvate can be completely oxidized to CO2, generating much more ATP, as will be described in chapters 17 and 18. Figure 16.1 shows some possible fates of pyruvate produced by glycolysis.
Because glucose is such a precious fuel, metabolic products, such as pyruvate and lactate, are salvaged to synthesize glucose in the process of gluconeogenesis. Although glycolysis and gluconeogenesis have some enzymes in common, the two pathways are not simply the reverse of each other. In particular, the highly exergonic, irreversible steps of glycolysis are bypassed in gluconeogenesis. The two pathways are reciprocally regulated so that glycolysis and gluconeogenesis do not take place simultaneously in the same cell to a significant extent.
Glycolysis
Derived from the Greek stem glyk-
450
Our understanding of glucose metabolism, especially glycolysis, has a rich history. Indeed, the development of biochemistry and the delineation of glycolysis went hand in hand. A key discovery was made by Hans and Eduard Buchner in 1897, quite by accident. The Buchners were interested in manufacturing cell-
Enzyme
A term coined by by Friedrich Wilhelm Kühne in 1878 to designate catalytically active substances that had formerly been called ferments. Derived from the Greek words en, “in,” and zyme, “leaven.”
Studies of muscle extracts then showed that many of the reactions of lactic acid fermentation were the same as those of alcoholic fermentation. This exciting discovery revealed an underlying unity in biochemistry. The complete glycolytic pathway was elucidated by 1940. Glycolysis is also known as the Embden–
Glucose is generated from dietary carbohydrates
We typically consume in our diets a generous amount of starch and a smaller amount of glycogen. These complex carbohydrates must be converted into simpler carbohydrates for absorption by the intestine and transport in the blood. Starch and glycogen are digested primarily by the pancreatic enzyme α-amylase and to a lesser extent by salivary α-amylase. Amylase cleaves the α-1,4 bonds of starch and glycogen, but not the α-1,6 bonds. The products are the di-
Maltase cleaves maltose into two glucose molecules, whereas α-glucosidase digests maltotriose and any other oligosaccharides that may have escaped digestion by the amylase. α-Dextrinase further digests the limit dextrin. Maltase and α-glucosidase are located on the surface of the intestinal cells, as is sucrase, an enzyme that degrades the sucrose contributed by vegetables to fructose and glucose. The enzyme lactase is responsible for degrading the milk sugar lactose into glucose and galactose. The monosaccharides are transported into the cells lining the intestine and then into the bloodstream.
451
Glucose is an important fuel for most organisms
 Glucose is a common and important fuel. In mammals, glucose is the only fuel that the brain uses under nonstarvation conditions and the only fuel that red blood cells can use at all. Indeed, almost all organisms use glucose, and most that do process it in a similar fashion. Recall from Chapter 11 that there are many carbohydrates. Why is glucose instead of some other monosaccharide such a prominent fuel? We can speculate on the reasons. First, glucose is one of several monosaccharides formed from formaldehyde under prebiotic conditions, and so it may have been available as a fuel source for primitive biochemical systems. Second, glucose has a low tendency, relative to other monosaccharides, to nonenzymatically glycosylate proteins. In their open-
Glucose is a common and important fuel. In mammals, glucose is the only fuel that the brain uses under nonstarvation conditions and the only fuel that red blood cells can use at all. Indeed, almost all organisms use glucose, and most that do process it in a similar fashion. Recall from Chapter 11 that there are many carbohydrates. Why is glucose instead of some other monosaccharide such a prominent fuel? We can speculate on the reasons. First, glucose is one of several monosaccharides formed from formaldehyde under prebiotic conditions, and so it may have been available as a fuel source for primitive biochemical systems. Second, glucose has a low tendency, relative to other monosaccharides, to nonenzymatically glycosylate proteins. In their open-