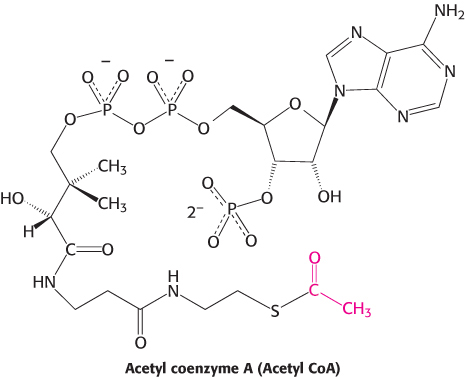
Roundabouts, or traffic circles, function as hubs to facilitate traffic flow. The citric acid cycle is the biochemical hub of the cell, oxidizing carbon fuels, usually in the form of acetyl CoA, as well as serving as a source of precursors for biosynthesis.
[(Left) Lynn Saville/Getty Images.]
The metabolism of glucose to pyruvate in glycolysis, an anaerobic process, harvests but a fraction of the ATP available from glucose. Most of the ATP generated in metabolism is provided by the aerobic processing of glucose. This process starts with the complete oxidation of glucose derivatives to carbon dioxide. This oxidation takes place in a series of reactions called the citric acid cycle, also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle. The citric acid cycle is the final common pathway for the oxidation of fuel molecules—carbohydrates, fatty acids, and amino acids. Most fuel molecules enter the cycle as acetyl coenzyme A.
Under aerobic conditions, the pyruvate generated from glucose is oxidatively decarboxylated to form acetyl CoA. In eukaryotes, the reactions of the citric acid cycle take place in the matrix of the mitochondria (Figure 17.1), in contrast with those of glycolysis, which take place in the cytoplasm.
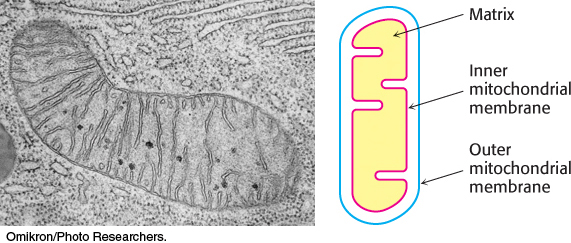
FIGURE 17.1Mitochondrion. The double membrane of the mitochondrion is evident in this electron micrograph. The numerous invaginations of the inner mitochondrial membrane are called cristae. The oxidative decarboxylation of pyruvate and the sequence of reactions in the citric acid cycle take place within the matrix.
[(Left) Omikron/Photo Researchers.]
The citric acid cycle harvests high-energy electrons
The citric acid cycle is the central metabolic hub of the cell. It is the gateway to the aerobic metabolism of any molecule that can be transformed into an acetyl group or a component of the citric acid cycle. The cycle is also an important source of precursors for the building blocks of many other molecules such as amino acids, nucleotide bases, and porphyrin (the organic component of heme). The citric acid cycle component, oxaloacetate, is also an important precursor to glucose (Section 16.3).
What is the function of the citric acid cycle in transforming fuel molecules into ATP? Recall that fuel molecules are carbon compounds that are capable of being oxidized—that is, of losing electrons (Chapter 15). The citric acid cycle includes a series of oxidation–reduction reactions that result in the oxidation of an acetyl group to two molecules of carbon dioxide. This oxidation generates high-energy electrons that will be used to power the synthesis of ATP. The function of the citric acid cycle is the harvesting of high-energy electrons from carbon fuels.
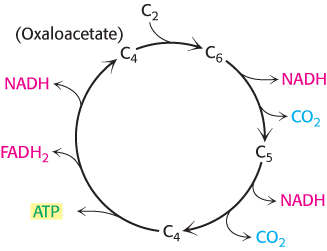
FIGURE 17.2Overview of the citric acid cycle. The citric acid cycle oxidizes two-carbon units, producing two molecules of CO2, one molecule of ATP, and high-energy electrons in the form of NADH and FADH2.
The overall pattern of the citric acid cycle is shown in Figure 17.2. A four-carbon compound (oxaloacetate) condenses with a two-carbon acetyl unit to yield a six-carbon tricarboxylic acid. The six-carbon compound releases CO2 twice in two successive oxidative decarboxylations that yield high-energy electrons. A four-carbon compound remains. This four-carbon compound is further processed to regenerate oxaloacetate, which can initiate another round of the cycle. Two carbon atoms enter the cycle as an acetyl unit and two carbon atoms leave the cycle in the form of two molecules of CO2.
Note that the citric acid cycle itself neither generates a large amount of ATP nor includes oxygen as a reactant (Figure 17.3). Instead, the citric acid cycle removes electrons from acetyl CoA and uses these electrons to reduce NAD+ and FAD to form NADH and FADH2. Three hydride ions (hence, six electrons) are transferred to three molecules of nicotinamide adenine dinucleotide (NAD+), and one pair of hydrogen atoms (hence, two electrons) is transferred to one molecule of flavin adenine dinucleotide (FAD). Electrons released in the reoxidation of NADH and FADH2 flow through a series of membrane proteins (referred to as the electron-transport chain) to generate a proton gradient across the inner mitochondrial membrane. These protons then flow through ATP synthase to generate ATP from ADP and inorganic phosphate. These electron carriers yield nine molecules of ATP when they are oxidized by O2 in oxidative phosphorylation (Chapter 18).
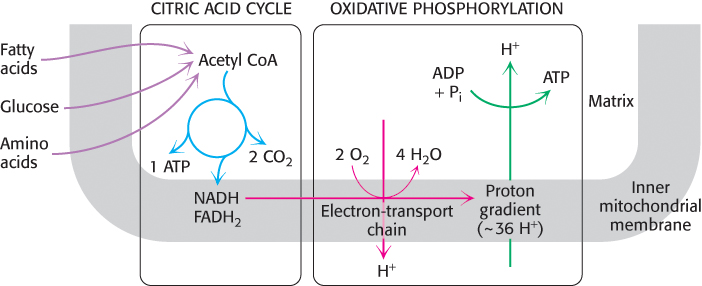
FIGURE 17.3Cellular respiration. The citric acid cycle constitutes the first stage in cellular respiration, the removal of high-energy electrons from carbon fuels in the form of NADH and FADH2 (blue pathway). These electrons reduce O2 to generate a proton gradient (red pathway), which is used to synthesize ATP (green pathway). The reduction of O2 and the synthesis of ATP constitute oxidative phosphorylation.
The citric acid cycle, in conjunction with oxidative phosphorylation, provides the preponderance of energy used by aerobic cells—in human beings, greater than 90%. It is highly efficient because the oxidation of a limited number of citric acid cycle molecules can generate large amounts of NADH and FADH2. Note in Figure 17.2 that the four-carbon molecule, oxaloacetate, that initiates the first step in the citric acid cycle is regenerated at the end of one passage through the cycle. Thus, one molecule of oxaloacetate is capable of participating in the oxidation of many acetyl molecules.