Oxidative Phosphorylation
CHAPTER
18
523
OUTLINE
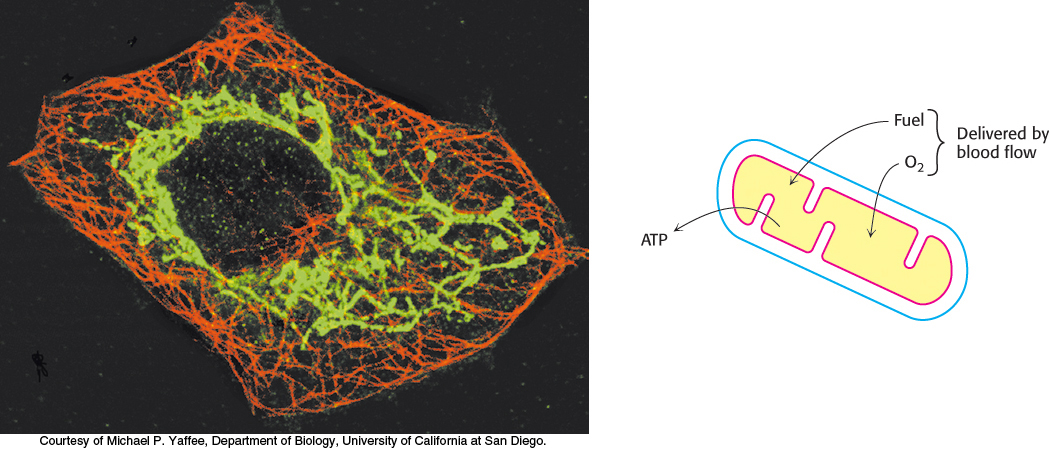
The amount of ATP that human beings require to go about their lives is staggering. A sedentary male of 70 kg (154 lbs) requires about 8400 kJ (2000 kcal) for a day’s worth of activity. To provide this much energy requires 83 kg of ATP. However, human beings possess only about 250 g of ATP at any given moment. The disparity between the amount of ATP that we have and the amount that we require is compensated by recycling ADP back to ATP. Each ATP molecule is recycled approximately 300 times per day. This recycling takes place primarily through oxidative phosphorylation.
We begin our study of oxidative phosphorylation by examining the oxidation–
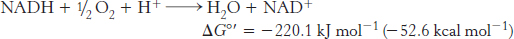
The overall reaction is exergonic. Importantly, three of the complexes of the electron-
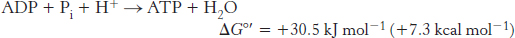
524
Thus, the oxidation of fuels and the phosphorylation of ADP are coupled by a proton gradient across the inner mitochondrial membrane (Figure 18.1).
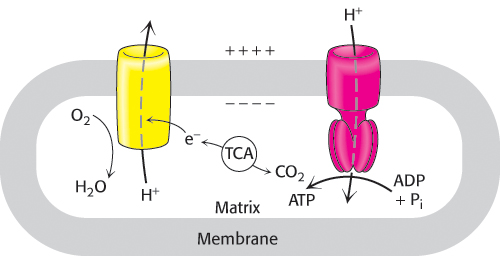
Respiration
An ATP-
Collectively, the generation of high-