The complete oxidation of glucose yields about 30 molecules of ATP
We can now estimate how many molecules of ATP are formed when glucose is completely oxidized to CO2. The number of ATP molecules formed in glycolysis and the citric acid cycle is unequivocally known because it is determined by the stoichiometries of chemical reactions. In contrast, the ATP yield of oxidative phosphorylation is less certain because the stoichiometries of proton pumping, ATP synthesis, and metabolite-transport processes need not be an integer or even have fixed values. As stated earlier, the best current estimates for the number of protons pumped out of the matrix by NADH-Q oxidoreductase, Q-cytochrome c oxidoreductase, and cytochrome c oxidase per electron pair are four, two, and four, respectively. The synthesis of a molecule of ATP is driven by the flow of about three protons through ATP synthase. An additional proton is consumed in transporting ATP from the matrix to the cytoplasm. Hence, about 2.5 molecules of cytoplasmic ATP are generated as a result of the flow of a pair of electrons from NADH to O2. For electrons that enter at the level of Q-cytochrome c oxidoreductase, such as those from the oxidation of succinate or cytoplasmic NADH transferred by the glycerol-phosphate shuttle, the yield is about 1.5 molecules of ATP per electron pair. Hence, as tallied in Table 18.4, about 30 molecules of ATP are formed when glucose is completely oxidized to CO2. Most of the ATP, 26 of 30 molecules formed, is generated by oxidative phosphorylation. Recall that the anaerobic metabolism of glucose yields only 2 molecules of ATP. One of the effects of endurance exercise, a practice that calls for much ATP for an extended period of time, is to increase the number of mitochondria and blood vessels in muscle and thus increase the extent of ATP generation by oxidative phosphorylation.
TABLE 18.4 ATP yield from the complete oxidation of glucose
|
|
ATP yield per glucose molecule |
Glycolysis: Conversion of glucose into pyruvate (in the cytoplasm) Phosphorylation of glucose Phosphorylation of fructose 6-phosphate Dephosphorylation of 2 molecules of 1,3-BPG Dephosphorylation of 2 molecules of phosphoenolpyruvate 2 molecules of NADH are formed in the oxidation of 2 molecules of glyceraldehyde 3-phosphate
|
|
Conversion of pyruvate into acetyl CoA (inside mitochondria) 2 molecules of NADH are formed
|
|
Citric acid cycle (inside mitochondria) 2 molecules of adenosine triphosphate are formed
from 2 molecules of succinyl CoA
6 molecules of NADH are formed in the oxidation of 2 molecules each of isocitrate, α-ketoglutarate, and malate
2 molecules of FADH2 are formed in the oxidation of 2 molecules of succinate
|
|
Oxidative phosphorylation (inside mitochondria) 2 molecules of NADH formed in glycolysis; each
yields 1.5 molecules of ATP (assuming transport of NADH by
the glycerol 3-phosphate shuttle)
2 molecules of NADH formed in the oxidative decarboxylation of pyruvate; each yields 2.5 molecules of ATP
2 molecules of FADH2 formed in the citric acid cycle; each yields 1.5 molecules of ATP
6 molecules of NADH formed in the citric acid cycle; each
yields 2.5 molecules of ATP
|
|
Net Yield per Molecule of Glucose |
|
Information on the ATP yield of oxidative phosphorylation is from values given in P. C. Hinkle, M. A. Kumar, A. Resetar, and D. L. Harris, Biochemistry 30:3576, 1991. Note: The current value of 30 molecules of ATP per molecule of glucose supersedes the earlier value of 36 molecules of ATP. The stoichiometries of proton pumping, ATP synthesis, and metabolite transport should be regarded as estimates. About 2 more molecules of ATP are formed per molecule of glucose oxidized when the malate–aspartate shuttle rather than the glycerol 3-phosphate shuttle is used. |
The rate of oxidative phosphorylation is determined by the need for ATP
How is the rate of the electron-transport chain controlled? Under most physiological conditions, electron transport is tightly coupled to phosphorylation. Electrons do not usually flow through the electron-transport chain to O2 unless ADP is simultaneously phosphorylated to ATP. When ADP concentration rises, as would be the case in active muscle, the rate of oxidative phosphorylation increases to meet the ATP needs of the muscle. The regulation of the rate of oxidative phosphorylation by the ADP level is called respiratory control or acceptor control. Experiments on isolated mitochondria demonstrate the importance of ADP level (Figure 18.40). The rate of oxygen consumption by mitochondria increases markedly when ADP is added and then returns to its initial value when the added ADP has been converted into ATP.
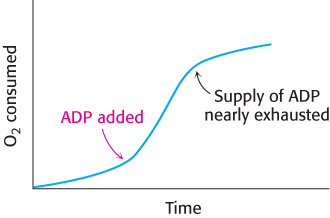
FIGURE 18.40Respiratory control. Electrons are transferred to O2 only if ADP is concomitantly phosphorylated to ATP.
The level of ADP likewise affects the rate of the citric acid cycle. At low concentrations of ADP, as in a resting muscle, NADH and FADH2 are not consumed by the electron-transport chain. The citric acid cycle slows because there is less NAD+ and FAD to feed the cycle. As the ADP level rises and oxidative phosphorylation speeds up, NADH and FADH2 are oxidized, and the citric acid cycle becomes more active. Electrons do not flow from fuel molecules to O2 unless ATP needs to be synthesized. We see here another example of the regulatory significance of the energy charge (Figure 18.41).
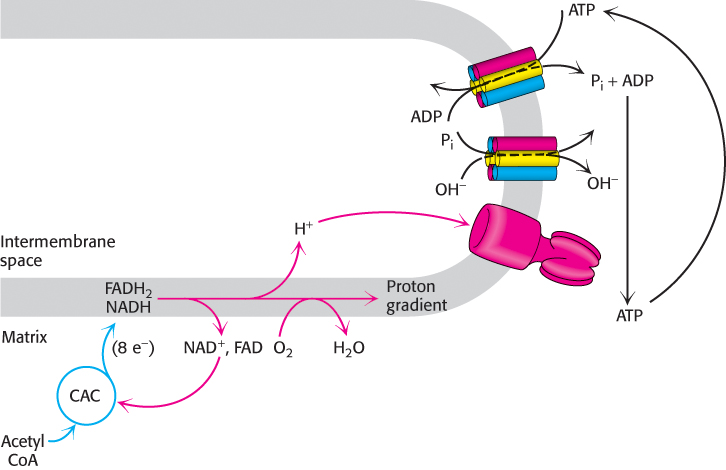
FIGURE 18.41Energy charge regulates the use of fuels. The synthesis of ATP from ADP and Pi controls the flow of electrons from NADH and FADH2 to oxygen. The availability of NAD+ and FAD in turn control the rate of the citric acid cycle (CAC).
ATP synthase can be regulated
Mitochondria contain an evolutionarily conserved protein, inhibitory factor 1 (IF1), that specifically inhibits the potential hydrolytic activity of the F0F1 ATP synthase. What is the function of IF1? Consider a circumstance where tissues may be deprived of oxygen (ischemia). Without oxygen as the electron acceptor, the electron transport chain will be unable to generate the proton-motive force. The ATP in the mitochondria would be hydrolyzed by the synthase, working in reverse (Problem 18.45). The role of IF1 is to prevent the wasteful hydrolysis of ATP by inhibiting the hydrolytic activity of the synthase.
 IF1 is overexpressed in many types of cancer. This over-expression plays a role in the induction of the Warburg effect, the switch from oxidative phosphorylation to aerobic glycolysis as the principle means for ATP synthesis (Section 16.2).
IF1 is overexpressed in many types of cancer. This over-expression plays a role in the induction of the Warburg effect, the switch from oxidative phosphorylation to aerobic glycolysis as the principle means for ATP synthesis (Section 16.2).
Regulated uncoupling leads to the generation of heat
Some organisms possess the ability to uncouple oxidative phosphorylation from ATP synthesis to generate heat. Such uncoupling is a means to maintain body temperature in hibernating animals, in some newborn animals (including human beings), and in many adult mammals, especially those adapted to cold. The skunk cabbage uses an analogous mechanism to heat its floral spikes in early spring, increasing the evaporation of odoriferous molecules that attract insects to fertilize its flowers. In animals, the uncoupling is in brown adipose tissue (BAT), which is specialized tissue for the process of nonshivering thermogenesis. In contrast, white adipose tissue (WAT), which constitutes the bulk of adipose tissue, plays no role in thermogenesis but serves as an energy source and an endocrine gland (Chapters 26 and 27).
Brown adipose tissue is very rich in mitochondria, often called brown fat mitochondria. The tissue appears brown from the combination of the greenish-colored cytochromes in the numerous mitochondria and the red hemoglobin present in the extensive blood supply, which helps to carry the heat through the body. The inner mitochondrial membrane of these mitochondria contains a large amount of uncoupling protein (UCP-1), or thermogenin, a dimer of 33-kDa subunits that resembles ATP-ADP translocase. UCP-1 transports protons from the cytoplasm to the matrix with the assistance of fatty acids. In essence, UCP-1 generates heat by short-circuiting the mitochondrial proton battery. The energy of the proton gradient, normally captured as ATP, is released as heat as the protons flow through UCP-1 to the mitochondrial matrix. This dissipative proton pathway is activated when the core body temperature begins to fall. In response to a temperature drop, α-adrenergic hormones stimulate the release of free fatty acids from triacylglycerols stored in cytoplasmic lipid granules (Section 22.2) (Figure 18.42). Long chain fatty acids bind to the cytoplasmic face of UCP-1, and the carboxyl group binds a proton. This causes a structural change in UCP-1 so that the protonated carboxyl now faces the proton-poor environment of the matrix, and the proton is released. Proton release resets UCP-1 to the initial state.
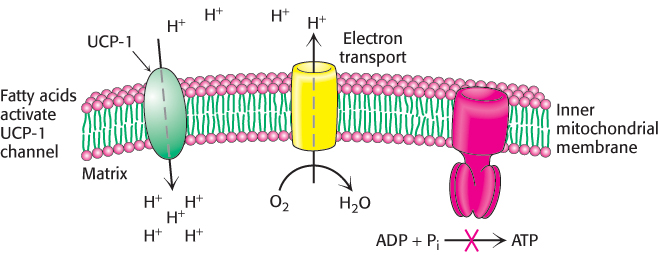
FIGURE 18.42Action of an uncoupling protein. Uncoupling protein (UCP-1) generates heat by permitting the influx of protons into the mitochondria without the synthesis of ATP.
 Until recently, adult humans were believed to lack brown fat tissue. However, new studies have established that adults, especially females, have brown adipose tissue in the neck and upper chest regions that is activated by cold (Figure 18.43). Obesity leads to a decrease in brown adipose tissue.
Until recently, adult humans were believed to lack brown fat tissue. However, new studies have established that adults, especially females, have brown adipose tissue in the neck and upper chest regions that is activated by cold (Figure 18.43). Obesity leads to a decrease in brown adipose tissue.
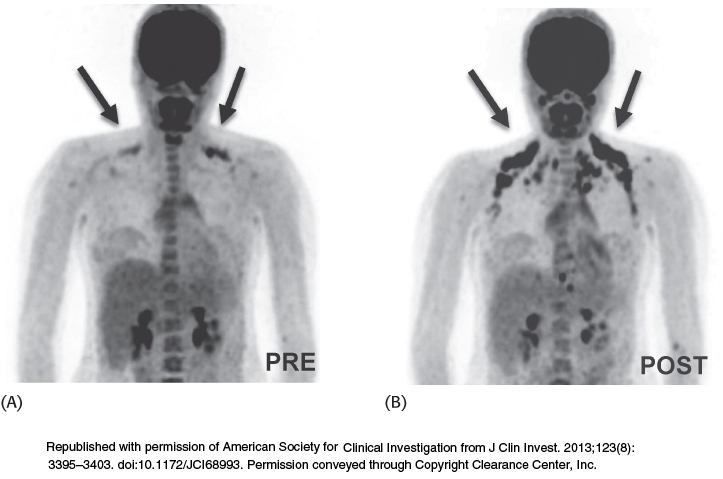
FIGURE 18.43Brown adipose tissue is revealed on exposure to cold. The results of PET–CT scanning show the uptake and distribution of18F-fluorodeoxyglucose (18F-FDG) in adipose tissue. The patterns of18F-FDG uptake in the same subject are dramatically different under thermoneutral conditions (A) and after exposure to cold (B).
[Republished with permission of American Society for Clinical Investigation from J Clin Invest. 2013;123(8):3395–3403. doi:10.1172/JCI68993. Permission conveyed through Copyright Clearance Center, Inc.]
 We can witness the effects of a lack of nonshivering thermogenesis by examining pig behavior. Pigs are unusual mammals in that they have large litters and are the only ungulates (hoofed animals) that build nests for birth. These behavioral characteristics appear to be the result of a biochemical deficiency. Pigs lack UCP-1 and, hence, brown fat. Piglets must rely on other means of thermogenesis, such as nesting, large litter size, and shivering.
We can witness the effects of a lack of nonshivering thermogenesis by examining pig behavior. Pigs are unusual mammals in that they have large litters and are the only ungulates (hoofed animals) that build nests for birth. These behavioral characteristics appear to be the result of a biochemical deficiency. Pigs lack UCP-1 and, hence, brown fat. Piglets must rely on other means of thermogenesis, such as nesting, large litter size, and shivering.
 In addition to UCP-1, two other uncoupling proteins have been identified. UCP-2, which is 56% identical in sequence with UCP-1, is found in a wide variety of tissues. UCP-3 (57% identical with UCP-1 and 73% identical with UCP-2) is localized to skeletal muscle and brown fat. This family of uncoupling proteins, especially UCP-2 and UCP-3, may play a role in energy homeostasis. In fact, the genes for UCP-2 and UCP-3 map to regions of the human and mouse chromosomes that have been linked to obesity, supporting the notion that they function as a means of regulating body weight.
In addition to UCP-1, two other uncoupling proteins have been identified. UCP-2, which is 56% identical in sequence with UCP-1, is found in a wide variety of tissues. UCP-3 (57% identical with UCP-1 and 73% identical with UCP-2) is localized to skeletal muscle and brown fat. This family of uncoupling proteins, especially UCP-2 and UCP-3, may play a role in energy homeostasis. In fact, the genes for UCP-2 and UCP-3 map to regions of the human and mouse chromosomes that have been linked to obesity, supporting the notion that they function as a means of regulating body weight.
Oxidative phosphorylation can be inhibited at many stages
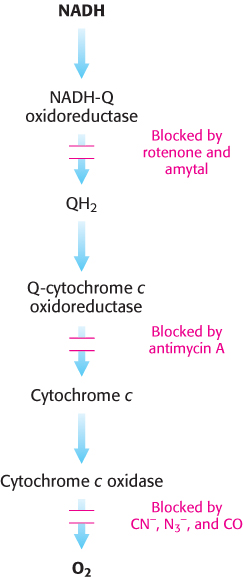
FIGURE 18.44Sites of action of some inhibitors of electron transport.
Many potent and lethal poisons exert their effect by inhibiting oxidative phosphorylation at one of a number of different locations (Figure 18.44):
1. Inhibition of the electron-transport chain. Rotenone, which is used as a fish and insect poison, and amytal, a barbiturate sedative, block electron transfer in NADH-Q oxidoreductase and thereby prevent the utilization of NADH as a substrate. Rotenone, as an electron-transport-chain inhibitor, may play a role, along with genetic susceptibility, in the development of Parkinson disease. In the presence of rotenone and amytal, electron flow resulting from the oxidation of succinate is unimpaired, because these electrons enter through QH2, beyond the block. Antimycin A interferes with electron flow from cytochrome bH in Q-cytochrome c oxidoreductase. Furthermore, electron flow in cytochrome c oxidase can be blocked by cyanide (CN−), azide (N3−), and carbon monoxide (CO). Cyanide and azide react with the ferric form of heme a3, whereas carbon monoxide inhibits the ferrous form. Inhibition of the electron-transport chain also inhibits ATP synthesis because the proton-motive force can no longer be generated.
2. Inhibition of ATP synthase. Oligomycin, an antibiotic used as an antifungal agent, and dicyclohexylcarbodiimide (DCC) prevent the influx of protons through ATP synthase by binding to the carboxylate group of the c subunits required for proton binding. Modification of only one c subunit by DCC is sufficient to inhibit the rotation of the entire c ring and hence ATP synthesis. If actively respiring mitochondria are exposed to an inhibitor of ATP synthase, the electron-transport chain ceases to operate. This observation clearly illustrates that electron transport and ATP synthesis are normally tightly coupled.
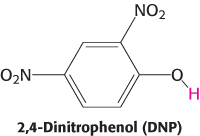
3. Uncoupling electron transport from ATP synthesis. The tight coupling of electron transport and phosphorylation in mitochondria can be uncoupled by 2,4-dinitrophenol (DNP) and certain other acidic aromatic compounds. These substances carry protons across the inner mitochondrial membrane, down their concentration gradient. In the presence of these uncouplers, electron transport from NADH to O2 proceeds in a normal fashion, but ATP is not formed by mitochondrial ATP synthase, because the proton-motive force across the inner mitochondrial membrane is continuously dissipated. This loss of respiratory control leads to increased oxygen consumption and oxidation of NADH. Indeed, in the accidental ingestion of uncouplers, large amounts of metabolic fuels are consumed, but no energy is captured as ATP. Rather, energy is released as heat. DNP is the active ingredient in some herbicides and fungicides. Remarkably, some people consume DNP as a weight-loss drug, despite the fact that the FDA banned its use in 1938. There are also reports that Soviet soldiers were given DNP to keep them warm during the long Russian winters. Chemical uncouplers are nonphysiological, unregulated counterparts of uncoupling proteins.
 Drugs are being sought that would function as mild uncouplers, uncouplers not as potentially lethal as DNP, for use in treatment of obesity and related pathologies. Xanthohumol, a prenylated chalcone found in hops and beer, shows promise in this regard. Xanthohumol also scavenges free radicals and is used for treatment of certain types of cancers.
Drugs are being sought that would function as mild uncouplers, uncouplers not as potentially lethal as DNP, for use in treatment of obesity and related pathologies. Xanthohumol, a prenylated chalcone found in hops and beer, shows promise in this regard. Xanthohumol also scavenges free radicals and is used for treatment of certain types of cancers.
4. Inhibition of ATP export. ATP-ADP translocase is specifically inhibited by very low concentrations of atractyloside (a plant glycoside) or bongkrekic acid (an antibiotic from a mold). Atractyloside binds to the translocase when its nucleotide site faces the cytoplasm, whereas bongkrekic acid binds when this site faces the mitochondrial matrix. Oxidative phosphorylation stops soon after either inhibitor is added, showing that ATP-ADP translocase is essential for maintaining adequate amounts of ADP to accept the energy associated with the proton-motive force.
Mitochondrial diseases are being discovered
 The number of diseases that can be attributed to mitochondrial mutations is steadily growing in step with our growing understanding of the biochemistry and genetics of mitochondria. The prevalence of mitochondrial diseases is estimated to be from 10 to 15 per 100,000 people, roughly equivalent to the prevalence of the muscular dystrophies. The first mitochondrial disease to be understood was Leber hereditary optic neuropathy (LHON), a form of blindness that strikes in midlife as a result of mutations in Complex I. Some of these mutations impair NADH utilization, whereas others block electron transfer to Q. Mutations in Complex I are the most frequent cause of mitochondrial diseases. The accumulation of mutations in mitochondrial genes in a span of several decades may contribute to aging, degenerative disorders, and cancer.
The number of diseases that can be attributed to mitochondrial mutations is steadily growing in step with our growing understanding of the biochemistry and genetics of mitochondria. The prevalence of mitochondrial diseases is estimated to be from 10 to 15 per 100,000 people, roughly equivalent to the prevalence of the muscular dystrophies. The first mitochondrial disease to be understood was Leber hereditary optic neuropathy (LHON), a form of blindness that strikes in midlife as a result of mutations in Complex I. Some of these mutations impair NADH utilization, whereas others block electron transfer to Q. Mutations in Complex I are the most frequent cause of mitochondrial diseases. The accumulation of mutations in mitochondrial genes in a span of several decades may contribute to aging, degenerative disorders, and cancer.
A human egg harbors several hundred thousand molecules of mitochondrial DNA, whereas a sperm contributes only a few hundred and thus has little effect on the mitochondrial genotype. Because the maternally inherited mitochondria are present in large numbers and not all of the mitochondria may be affected, the pathologies of mitochondrial mutants can be quite complex. Even within a single family carrying an identical mutation, chance fluctuations in the percentage of mitochondria with the mutation lead to large variations in the nature and severity of the symptoms of the pathological condition as well as the time of onset. As the percentage of defective mitochondria increases, energy-generating capacity diminishes until, at some threshold, the cell can no longer function properly. Defects in cellular respiration are doubly dangerous. Not only does energy transduction decrease, but also the likelihood that reactive oxygen species will be generated increases. Organs that are highly dependent on oxidative phosphorylation, such as the nervous system, the retina, and the heart, are most vulnerable to mutations in mitochondrial DNA.
Mitochondria play a key role in apoptosis
In the course of development or in cases of significant cell damage, individual cells within multicellular organisms undergo programmed cell death, or apoptosis. Mitochondria act as control centers regulating this process. Although the details have not yet been established, the outer membrane of damaged mitochondria becomes highly permeable, a process referred to as mitochondrial outer membrane permeabilization (MOMP). This permeabilization is instigated by a family of proteins (Bcl family) that were initially discovered because of their role in cancer. One of the most potent activators of apoptosis, cytochrome c, exits the mitochondria and interacts with apoptotic peptidase-activating factor 1 (APAF-1), which leads to the formation of the apoptosome. The apoptosome recruits and activates a proteolytic enzyme called caspase 9, a member of the cysteine protease family (Section 9.1) that in turn activates a cascade of other caspases. Each caspase type destroys a particular target, such as the proteins that maintain cell structure. Another target is a protein that inhibits an enzyme that destroys DNA (an enzyme called caspase-activated DNAse or CAD), freeing CAD to cleave the genetic material. This cascade of proteolytic enzymes has been called “death by a thousand tiny cuts.”
Power transmission by proton gradients is a central motif of bioenergetics
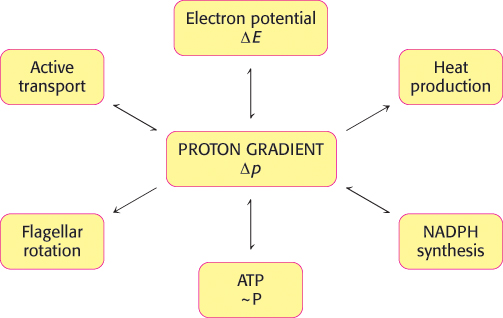
FIGURE 18.45The proton gradient is an interconvertible form of free energy.
The main concept presented in this chapter is that mitochondrial electron transfer and ATP synthesis are linked by a transmembrane proton gradient. ATP synthesis in bacteria and chloroplasts also is driven by proton gradients. In fact, proton gradients power a variety of energy-requiring processes such as the active transport of calcium ions by mitochondria, the entry of some amino acids and sugars into bacteria, the rotation of bacterial flagella, and the transfer of electrons from NADP+ to NADPH. Proton gradients can also be used to generate heat, as in nonshivering thermogenesis. It is evident that proton gradients are a central interconvertible currency of free energy in biological systems (Figure 18.45). Mitchell noted that the proton-motive force is a marvelously simple and effective store of free energy because it requires only a thin, closed lipid membrane between two aqueous phases.


 IF1 is overexpressed in many types of cancer. This over-
IF1 is overexpressed in many types of cancer. This over-
 Until recently, adult humans were believed to lack brown fat tissue. However, new studies have established that adults, especially females, have brown adipose tissue in the neck and upper chest regions that is activated by cold (Figure 18.43). Obesity leads to a decrease in brown adipose tissue.
Until recently, adult humans were believed to lack brown fat tissue. However, new studies have established that adults, especially females, have brown adipose tissue in the neck and upper chest regions that is activated by cold (Figure 18.43). Obesity leads to a decrease in brown adipose tissue.
 We can witness the effects of a lack of nonshivering thermogenesis by examining pig behavior. Pigs are unusual mammals in that they have large litters and are the only ungulates (hoofed animals) that build nests for birth. These behavioral characteristics appear to be the result of a biochemical deficiency. Pigs lack UCP-
We can witness the effects of a lack of nonshivering thermogenesis by examining pig behavior. Pigs are unusual mammals in that they have large litters and are the only ungulates (hoofed animals) that build nests for birth. These behavioral characteristics appear to be the result of a biochemical deficiency. Pigs lack UCP- In addition to UCP-
In addition to UCP-

 Drugs are being sought that would function as mild uncouplers, uncouplers not as potentially lethal as DNP, for use in treatment of obesity and related pathologies. Xanthohumol, a prenylated chalcone found in hops and beer, shows promise in this regard. Xanthohumol also scavenges free radicals and is used for treatment of certain types of cancers.
Drugs are being sought that would function as mild uncouplers, uncouplers not as potentially lethal as DNP, for use in treatment of obesity and related pathologies. Xanthohumol, a prenylated chalcone found in hops and beer, shows promise in this regard. Xanthohumol also scavenges free radicals and is used for treatment of certain types of cancers. The number of diseases that can be attributed to mitochondrial mutations is steadily growing in step with our growing understanding of the biochemistry and genetics of mitochondria. The prevalence of mitochondrial diseases is estimated to be from 10 to 15 per 100,000 people, roughly equivalent to the prevalence of the muscular dystrophies. The first mitochondrial disease to be understood was Leber hereditary optic neuropathy (LHON), a form of blindness that strikes in midlife as a result of mutations in Complex I. Some of these mutations impair NADH utilization, whereas others block electron transfer to Q. Mutations in Complex I are the most frequent cause of mitochondrial diseases. The accumulation of mutations in mitochondrial genes in a span of several decades may contribute to aging, degenerative disorders, and cancer.
The number of diseases that can be attributed to mitochondrial mutations is steadily growing in step with our growing understanding of the biochemistry and genetics of mitochondria. The prevalence of mitochondrial diseases is estimated to be from 10 to 15 per 100,000 people, roughly equivalent to the prevalence of the muscular dystrophies. The first mitochondrial disease to be understood was Leber hereditary optic neuropathy (LHON), a form of blindness that strikes in midlife as a result of mutations in Complex I. Some of these mutations impair NADH utilization, whereas others block electron transfer to Q. Mutations in Complex I are the most frequent cause of mitochondrial diseases. The accumulation of mutations in mitochondrial genes in a span of several decades may contribute to aging, degenerative disorders, and cancer.