18.3
The Respiratory Chain Consists of Four Complexes: Three Proton Pumps and a Physical Link to the Citric Acid Cycle
Electrons are transferred from NADH to O2 through a chain of three large protein complexes called NADH-Q oxidoreductase, Q-cytochrome c oxidoreductase, and cytochrome c oxidase (Figure 18.6 and Table 18.2). Electron flow within these transmembrane complexes is highly exergonic and powers the transport of protons across the inner mitochondrial membrane. A fourth large protein complex, called succinate-Q reductase, contains the succinate dehydrogenase that generates FADH2 in the citric acid cycle. Electrons from this FADH2 enter the electron-transport chain at Q-cytochrome c oxidoreductase. Succinate-Q reductase, in contrast with the other complexes, does not pump protons. NADH-Q oxidoreductase, succinate-Q reductase, Q-cytochrome c oxidoreductase, and cytochrome c oxidase are also called Complex I, II, III, and IV, respectively. Complexes I, III, and IV appear to be associated in a supramolecular complex termed the respirasome. As we have seen before, such supramolecular complexes facilitate the rapid transfer of substrate and prevent the release of reaction intermediates.
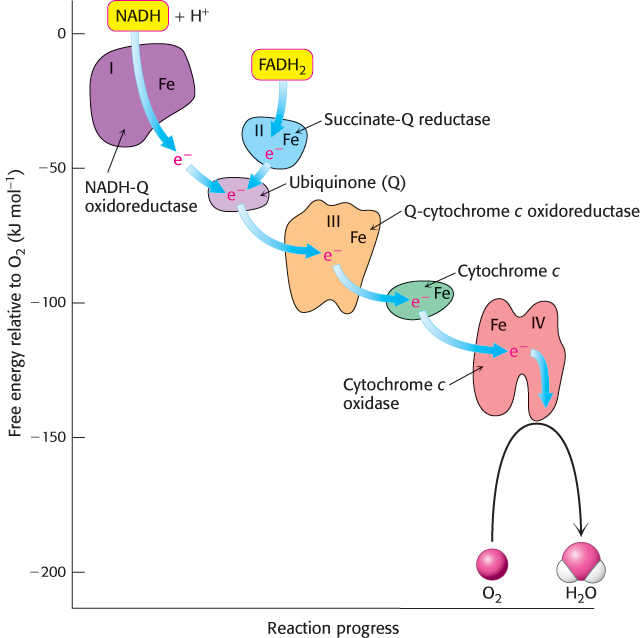
FIGURE 18.6Components of the electron-transport chain. Electrons flow down an energy gradient from NADH to O2. The flow is catalyzed by four protein complexes, and the energy released is used to generate a proton gradient.
[Information from D. Sadava et al., Life, 8th ed. (Sinauer, 2008), p. 150.]
TABLE 18.2 Components of the mitochondrial electron-transport chain
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q-cytochrome c oxidoreductase |
|
|
Heme bL
Heme c1
Fe-S
|
|
|
|
|
|
|
|
Heme a3
CuA and CuB
|
|
|
|
Information from: J. W. DePierre and L. Ernster, Annu. Rev. Biochem. 46:215, 1977; Y. Hatefi, Annu Rev. Biochem. 54:1015, 1985; and J. E. Walker, Q. Rev. Biophys. 25:253, 1992. |
Two special electron carriers ferry the electrons from one complex to the next. The first is coenzyme Q (Q), also known as ubiquinone because it is a ubiquitous quinone in biological systems. Ubiquinone is a hydrophobic quinone that diffuses rapidly within the inner mitochondrial membrane. Electrons are carried from NADH-Q oxidoreductase to Q-cytochrome c oxidoreductase, the third complex of the chain, by the reduced form of Q. Electrons from the FADH2 generated by the citric acid cycle are transferred first to ubiquinone and then to the Q-cytochrome c oxidoreductase complex.
Coenzyme Q is a quinone derivative with a long tail consisting of five-carbon isoprene units that account for its hydrophobic nature. The number of isoprene units in the tail depends on the species. The most common mammalian form contains 10 isoprene units (coenzyme Q10). For simplicity, the subscript will be omitted from this abbreviation because all varieties function in an identical manner. Quinones can exist in several oxidation states. In the fully oxidized state (Q), coenzyme Q has two keto groups (Figure 18.7). The addition of one electron and one proton results in the semiquinone form (QH·). The semiquinone can lose a proton to form a semiquinone radical anion (Q·−). The addition of a second electron and proton to the semiquinone generates ubiquinol (QH2), the fully reduced form of coenzyme Q, which holds its protons more tightly. Thus, for quinones, electron-transfer reactions are coupled to proton binding and release, a property that is key to transmembrane proton transport. Because ubiquinone is soluble in the membrane, a pool of Q and QH2—the Q pool—is thought to exist in the inner mitochondrial membrane, although recent research suggests that the Q pool is confined to the respirasome.
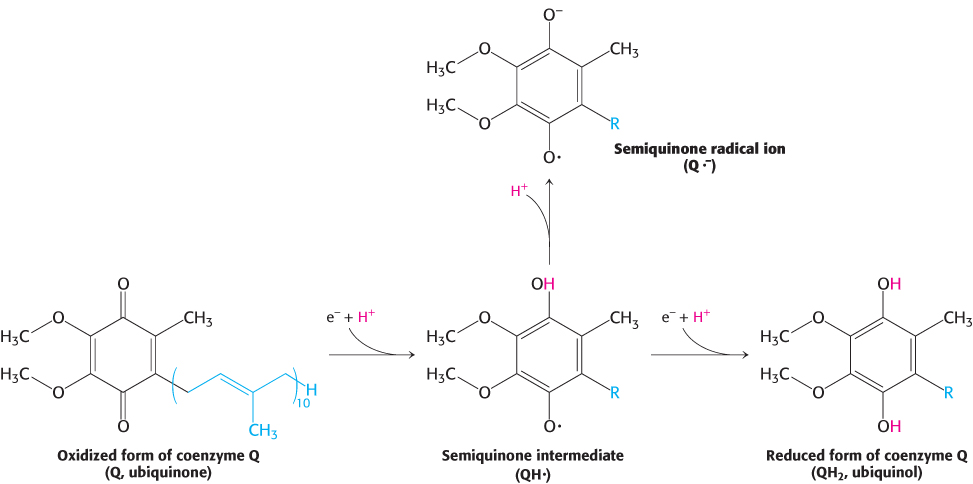
FIGURE 18.7Oxidation states of quinones. The reduction of ubiquinone (Q) to ubiquinol (QH2) proceeds through a semiquinone intermediate (QH·).
In contrast with Q, the second special electron carrier is a protein. Cytochrome c, a small soluble protein, shuttles electrons from Q-cytochrome c oxidoreductase to cytochrome c oxidase, the final component in the chain and the one that catalyzes the reduction of O2.
Iron–sulfur clusters are common components of the electron transport chain
Iron–sulfur clusters in iron–sulfur proteins (also called nonheme iron proteins) play a critical role in a wide range of reduction reactions in biological systems. Several types of Fe-S clusters are known (Figure 18.8a). In the simplest kind, a single iron ion is tetrahedrally coordinated to the sulfhydryl groups of four cysteine residues of the protein (Figure 18.8). A second kind, denoted by 2Fe-2S, contains two iron ions, two inorganic sulfides, and usually four cysteine residues (Figure 18.8b). A third type, designated 4Fe-4S, contains four iron ions, four inorganic sulfides, and four cysteine residues (Figure 18.8c). NADH-Q oxidoreductase contains both 2Fe-2S and 4Fe-4S clusters. Iron ions in these Fe-S complexes cycle between Fe2+ (reduced) and Fe3+ (oxidized) states. Unlike quinones and flavins, iron–sulfur clusters generally undergo oxidation–reduction reactions without releasing or binding protons.
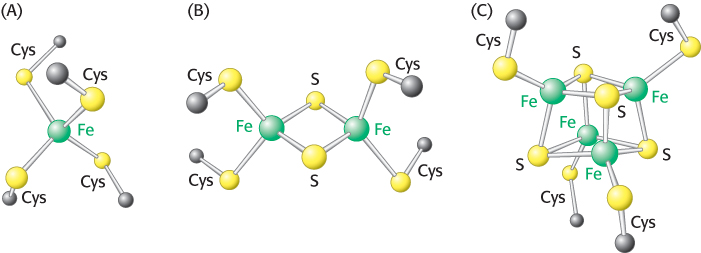
FIGURE 18.8Iron–sulfur clusters. (A) A single iron ion bound by four cysteine residues. (B) 2Fe-2S cluster with iron ions bridged by sulfide ions. (C) 4Fe-4S cluster. Each of these clusters can undergo oxidation–reduction reactions.
 The importance of Fe-S clusters is illustrated by the loss of function of the protein frataxin. Frataxin is a small (14.2-kDa) mitochondrial protein that is crucial for the synthesis of Fe-S clusters. Mutations in frataxin causes Friedreich’s ataxia, a disease of varying severity that affects the central and peripheral nervous as well as the heart and skeletal system. Severe cases lead to death in young adult life. The most common mutation is trinucleotide expansion (Section 28.5) in the gene for frataxin.
The importance of Fe-S clusters is illustrated by the loss of function of the protein frataxin. Frataxin is a small (14.2-kDa) mitochondrial protein that is crucial for the synthesis of Fe-S clusters. Mutations in frataxin causes Friedreich’s ataxia, a disease of varying severity that affects the central and peripheral nervous as well as the heart and skeletal system. Severe cases lead to death in young adult life. The most common mutation is trinucleotide expansion (Section 28.5) in the gene for frataxin.
The high-potential electrons of NADH enter the respiratory chain at NADH-Q oxidoreductase
The electrons of NADH enter the chain at NADH-Q oxidoreductase (also called Complex I and NADH dehydrogenase), an enormous enzyme (> 900 kDa) consisting of approximately 46 polypeptide chains. This proton pump, like that of the other two in the respiratory chain, is encoded by genes residing in both the mitochondria and the nucleus. NADH-Q oxidoreductase is L-shaped, with a horizontal arm lying in the membrane and a vertical arm that projects into the matrix.
The reaction catalyzed by this enzyme appears to be
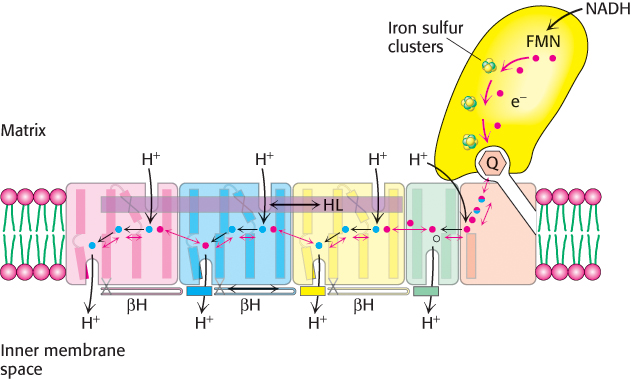
FIGURE 18.10Coupled electron–proton transfer reactions through NADH-Q oxidoreductase. Electrons flow in Complex I from NADH through FMN and a series of iron–sulfur clusters to ubiquinone (Q), forming Q2-. The charges on Q2- are electrostatically transmitted to hydrophilic amino acid residues (shown as red (glutamate) and blue (lysine or histidine) balls) that power the movement of HL and βH components. This movement changes the conformation of the transmembrane helices and results in the transport of four protons out of the mitochondrial matrix.
[Information from R. Baradaran et al., Nature 494:443–448.]
The initial step is the binding of NADH and the transfer of its two high-potential electrons to the flavin mononucleotide (FMN) prosthetic group, yielding the reduced form, FMNH2 (Figure 18.9). The electron acceptor of FMN, the isoalloxazine ring, is identical with that of FAD. Electrons are then transferred from FMNH2 to a series of iron–sulfur clusters, the second type of prosthetic group in NADH-Q oxidoreductase.
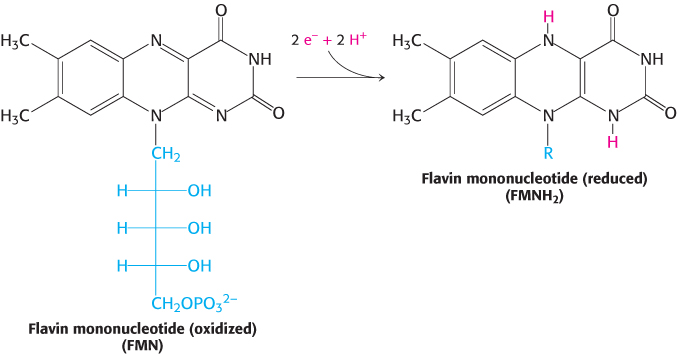
FIGURE 18.9Oxidation states of flavins.
Recent structural studies have suggested how Complex I acts as a proton pump. What are the structural elements required for proton-pumping? The membrane-embedded part of the complex has four proton half-channels consisting, in part, of vertical helices. One set of half-channels is exposed to the matrix and the other to the inner membrane space (Figure 18.10). The vertical helices are linked on the matrix side by a long horizontal helix (HL) that connects the matrix half-channels, while the cytoplasmic half-channels are joined by a series of β-hairpin-helix connecting elements (βH). An enclosed Q chamber, the site where Q accepts electrons from NADH, exists near the junction of the hydrophilic portion and the membrane-embedded portion. Finally, a hydrophilic funnel connects the Q chamber to a water-lined channel (into which the half channels open) that extends the entire length of the membrane-embedded portion.
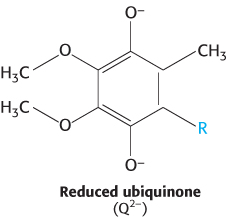
How do these structural elements cooperate to pump protons out of the matrix? When Q accepts two electrons from NADH, generating Q2−, the negative charges on Q2− interact electrostatically with negatively-charged amino acid residues in the membrane-embedded arm, causing conformational changes in the long horizontal helix and the βH elements. These changes in turn alter the structures of the connected vertical helices that change the pKa of amino acids, allowing protons from the matrix to first bind to the amino acids, then dissociate into the water-lined channel and finally enter the intermembrane space. Thus, the flow of two electrons from NADH to coenzyme Q through NADH-Q oxidoreductase leads to the pumping of four hydrogen ions out of the matrix of the mitochondrion. Q2− subsequently takes up two protons from the matrix as it is reduced to QH2. The removal of these protons from the matrix contributes to the formation of the proton motive force. The QH2 subsequently leaves the enzyme for the Q pool, allowing another reaction cycle to occur.
It is important to note that the citric acid cycle is not the only source of mitochondrial NADH. As we will see in Chapter 22, fatty acid degradation, which also takes place in mitochondria, is another crucial source of NADH for the electron-transport chain. Moreover, electrons from cytoplasmically generated NADH can be transported into mitochondria for use by the electron-transport chain (Section 18.5).
Ubiquinol is the entry point for electrons from FADH2 of flavoproteins
FADH2 enters the electron-transport chain at the second protein complex of the chain. Recall that FADH2 is formed in the citric acid cycle, in the oxidation of succinate to fumarate by succinate dehydrogenase (Section 17.2). Succinate dehydrogenase is part of the succinate-Q reductase complex (Complex II), an integral membrane protein of the inner mitochondrial membrane. FADH2 does not leave the complex. Rather, its electrons are transferred to Fe-S centers and then finally to Q to form QH2, which then is ready to transfer electrons further down the electron-transport chain. The succinate-Q reductase complex, in contrast with NADH-Q oxidoreductase, does not pump protons from one side of the membrane to the other. Consequently, less ATP is formed from the oxidation of FADH2 than from NADH.
Electrons flow from ubiquinol to cytochrome c through Q-cytochrome c oxidoreductase
What is the fate of ubiquinol generated by Complexes I and II? The electrons from QH2 are passed on to cytochrome c (Cyt c), a water-soluble protein, by the second of the three proton pumps in the respiratory chain, Q-cytochrome c oxidoreductase (also known as Complex III and as cytochrome c reductase). The flow of a pair of electrons through this complex leads to the effective net transport of 2 H+ to the cytoplasmic side, half the yield obtained with NADH-Q reductase because of a smaller thermodynamic driving force.
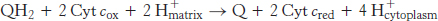
Q-cytochrome c oxidoreductase itself contains two types of cytochromes, named b and c1 (Figure 18.11). A cytochrome is an electron-transferring protein that contains a heme prosthetic group. The heme prosthetic group in cytochromes b, c1, and c is iron-protoporphyrin IX, the same heme present in myoglobin and hemoglobin (Section 7.1). The iron ion of a cytochrome, in contrast to hemoglobin and myoglobin, alternates between a reduced ferrous (+2) state and an oxidized ferric (+3) state during electron transport. The two cytochrome subunits of Q-cytochrome c oxidoreductase contain a total of three hemes: two hemes within cytochrome b, termed heme bL (L for low affinity) and heme bH (H for high affinity), and one heme within cytochrome c1. These identical hemes have different electron affinities because they are in different polypeptide environments. For example, heme bL, which is located in a cluster of helices near the cytoplasmic face of the membrane, has lower affinity for an electron than does heme bH, which is near the matrix side.
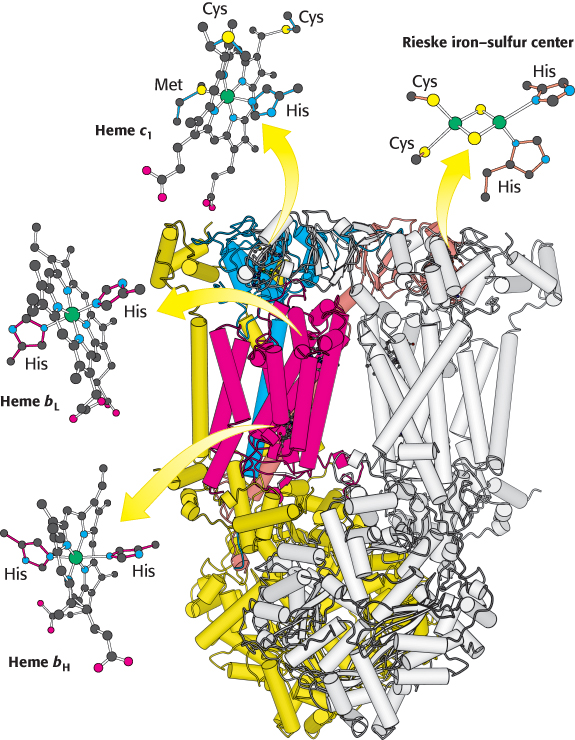
 FIGURE 18.11 Structure of Q-cytochrome c oxidoreductase. This enzyme is a homodimer with each monomer consisting of 11 distinct polypeptide chains. Some of the more prominent components in one monomer are colored while the other monomer is white. Although each monomer contains the same components, some are identified in one monomer or the other for ease of viewing. Notice that the major prosthetic groups, three hemes and a 2Fe-2S cluster, are located either near the cytoplasmic edge of the complex bordering the intermembrane space (top) or in the region embedded in the membrane (α helices represented by tubes). They are well positioned to mediate the electron-transfer reactions between quinones in the membrane and cytochrome c in the intermembrane space.
FIGURE 18.11 Structure of Q-cytochrome c oxidoreductase. This enzyme is a homodimer with each monomer consisting of 11 distinct polypeptide chains. Some of the more prominent components in one monomer are colored while the other monomer is white. Although each monomer contains the same components, some are identified in one monomer or the other for ease of viewing. Notice that the major prosthetic groups, three hemes and a 2Fe-2S cluster, are located either near the cytoplasmic edge of the complex bordering the intermembrane space (top) or in the region embedded in the membrane (α helices represented by tubes). They are well positioned to mediate the electron-transfer reactions between quinones in the membrane and cytochrome c in the intermembrane space.
[Drawn from 1BCC.pdb.]
In addition to the hemes, the enzyme contains an iron–sulfur protein with a 2Fe-2S center. This center, termed the Rieske center, is unusual in that one of the iron ions is coordinated by two histidine residues rather than two cysteine residues. This coordination stabilizes the center in its reduced form, raising its reduction potential so that it can readily accept electrons from QH2.
The Q cycle funnels electrons from a two-electron carrier to a one-electron carrier and pumps protons
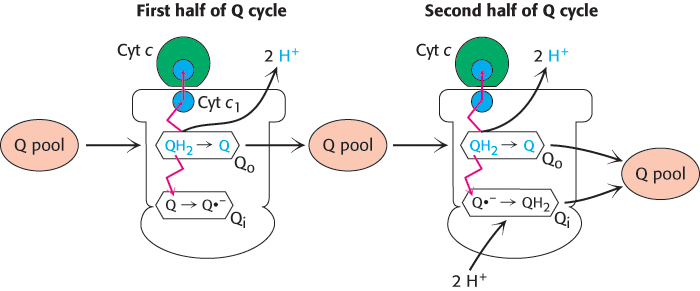
FIGURE 18.12Q cycle. The Q cycle takes place in Complex III, which is represented in outline form. In the first half of the cycle, two electrons of a bound QH2 are transferred, one to cytochrome c and the other to a bound Q in a second binding site to form the semiquinone radical anion Q·−. The newly formed Q dissociates and enters the Q pool. In the second half of the cycle, a second QH2 also gives up its electrons to Complex III, one to a second molecule of cytochrome c and the other to reduce Q·− to QH2. This second electron transfer results in the uptake of two protons from the matrix. The path of electron transfer is shown in red.
QH2 passes two electrons to Q-cytochrome c oxidoreductase, but the acceptor of electrons in this complex, cytochrome c, can accept only one electron. How does the switch from the two-electron carrier ubiquinol to the one-electron carrier cytochrome c take place? The mechanism for the coupling of electron transfer from Q to cytochrome c to transmembrane proton transport is known as the Q cycle (Figure 18.12). Two QH2 molecules bind to the complex consecutively, each giving up two electrons and two H+. These protons are released to the cytoplasmic side of the membrane. The first QH2 to exit the Q pool binds to the first Q binding site (Q o), and its two electrons travel through the complex to different destinations. One electron flows, first, to the Rieske 2Fe-2S cluster; then, to cytochrome c1 ; and, finally, to a molecule of oxidized cytochrome c, converting it into its reduced form. The reduced cytochrome c molecule is free to diffuse away from the enzyme to continue down the respiratory chain.
The second electron passes through two heme groups of cytochrome b to an oxidized ubiquinone in a second Q binding site (Qi).The Q in the second binding site is reduced to a semiquinone radical anion (Q·−) by the electron from the first QH2. The now fully oxidized Q leaves the first Q site, free to reenter the Q pool.
A second molecule of QH2 binds to the Qo site of Q-cytochrome c oxidoreductase and reacts in the same way as the first. One of the electrons is transferred to cytochrome c and the second electron is transferred to the partly reduced ubiquinone bound in the Qi binding site. On the addition of the electron from the second QH2 molecule, this quinone radical anion takes up two protons from the matrix side to form QH2. The removal of these two protons from the matrix contributes to the formation of the proton gradient. In sum, four protons are released on the cytoplasmic side, and two protons are removed from the mitochondrial matrix.
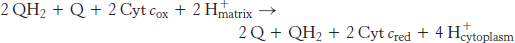
In one Q cycle, two QH2 molecules are oxidized to form two Q molecules, and then one Q molecule is reduced to QH2. The problem of how to efficiently funnel electrons from a two-electron carrier (QH2) to a one-electron carrier (cytochrome c) is solved by the Q cycle. The cytochrome b component of the reductase is in essence a recycling device that enables both electrons of QH2 to be used effectively.
Cytochrome c oxidase catalyzes the reduction of molecular oxygen to water
The last of the three proton-pumping assemblies of the respiratory chain is cytochrome c oxidase (Complex IV). Cytochrome c oxidase catalyzes the transfer of electrons from the reduced form of cytochrome c to molecular oxygen, the final acceptor.
The requirement of oxygen for this reaction is what makes “aerobic” organisms aerobic. To obtain oxygen for this reaction is the reason that human beings must breathe. Four electrons are funneled to O2 to completely reduce it to H2O, and, concomitantly, protons are pumped from the matrix to the cytoplasmic side of the inner mitochondrial membrane. This reaction is quite thermodynamically favorable. From the reduction potentials in Table 18.1, the standard free-energy change for this reaction is calculated to be ΔG°′ = −231.8 kJ mol−1 (−55.4 kcal mol−1). As much of this free energy as possible must be captured in the form of a proton gradient for subsequent use in ATP synthesis.
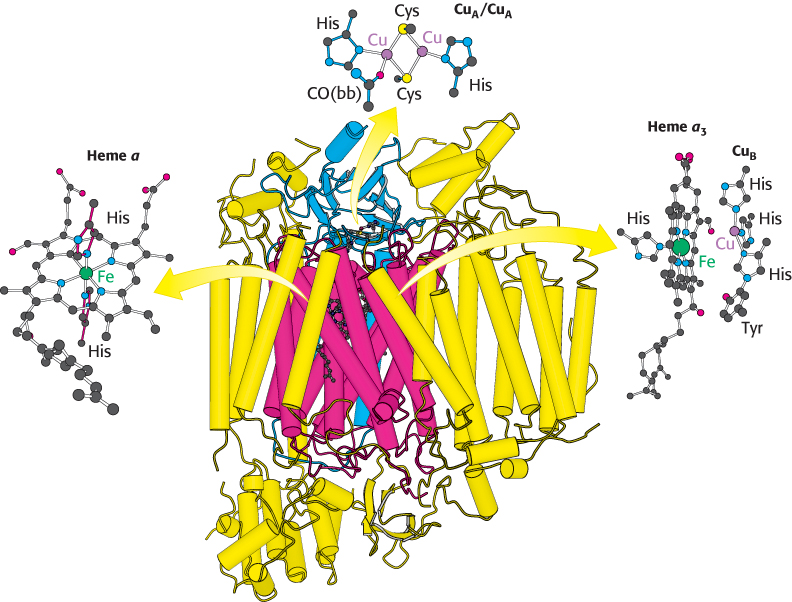
 FIGURE 18.13 Structure of cytochrome c oxidase. This enzyme consists of 13 polypeptide chains. Notice that most of the complex, as well as two major prosthetic groups (heme a and heme a3–CuB) are embedded in the membrane (α helices represented by vertical tubes). Heme a3–CuB is the site of the reduction of oxygen to water. The CuA/CuA prosthetic group is positioned near the intermembrane space to better accept electrons from cytochrome c. CO(bb) is a carbonyl group of the peptide backbone.
FIGURE 18.13 Structure of cytochrome c oxidase. This enzyme consists of 13 polypeptide chains. Notice that most of the complex, as well as two major prosthetic groups (heme a and heme a3–CuB) are embedded in the membrane (α helices represented by vertical tubes). Heme a3–CuB is the site of the reduction of oxygen to water. The CuA/CuA prosthetic group is positioned near the intermembrane space to better accept electrons from cytochrome c. CO(bb) is a carbonyl group of the peptide backbone.
[Drawn from 2OCC.pdb.]
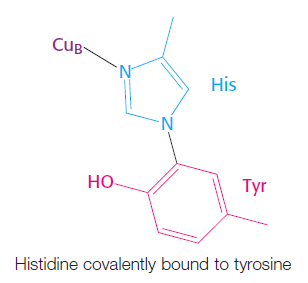
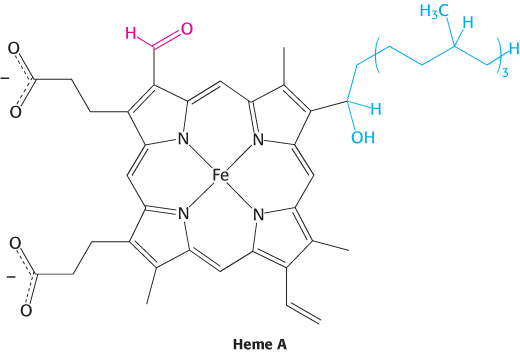
Bovine cytochrome c oxidase is reasonably well understood at the structural level (Figure 18.13). It consists of 13 subunits, 3 of which are encoded by the mitochondrion’s own genome. Cytochrome c oxidase contains two heme A groups and three copper ions, arranged as two copper centers, designated A and B. One center, CuA/CuA, contains two copper ions linked by two bridging cysteine residues. This center initially accepts electrons from reduced cytochrome c. The remaining copper ion, CuB, is bonded to three histidine residues, one of which is modified by covalent linkage to a tyrosine residue. The copper centers alternate between the reduced Cu+ (cuprous) form and the oxidized Cu2+ (cupric) form as they accept and donate electrons.
There are two heme A molecules, called heme a and heme a3, in cytochrome c oxidase. Heme A differs from the heme in cytochrome c and c1 in three ways: (1) a formyl group replaces a methyl group, (2) a C17 hydrocarbon chain replaces one of the vinyl groups, and (3) the heme is not covalently attached to the protein.
Heme a and heme a3 have distinct redox potentials because they are located in different environments within cytochrome c oxidase. An electron flows from cytochrome c to CuA/CuA, to heme a, to heme a3, to CuB, and finally to O2. Heme a3 and CuB are directly adjacent. Together, heme a3 and CuB form the active center at which O2 is reduced to H2O.
Four molecules of cytochrome c bind consecutively to the enzyme and transfer an electron to reduce one molecule of O2 to H2O (Figure 18.14):
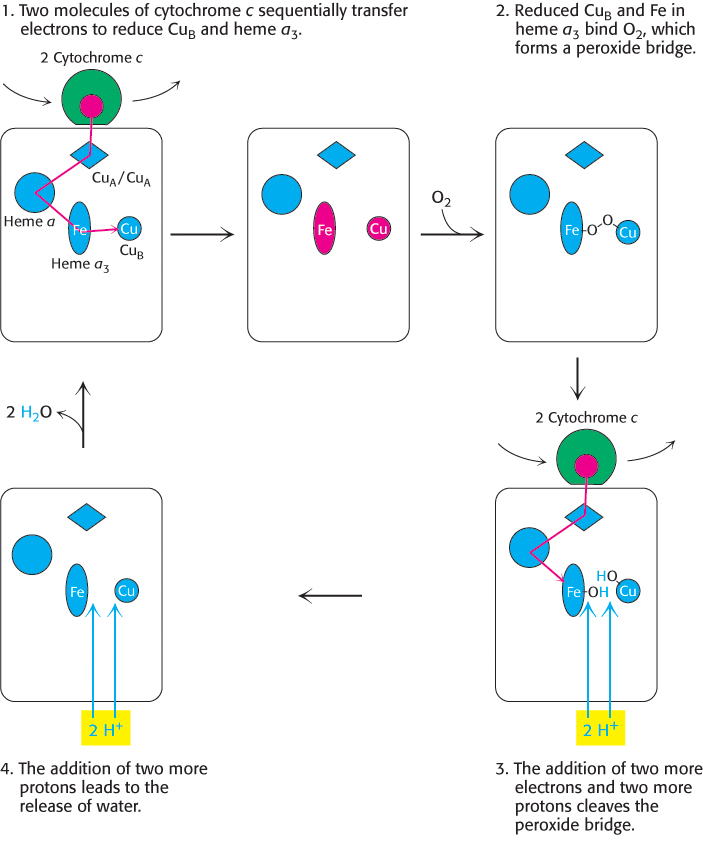
FIGURE 18.14Cytochrome c oxidase mechanism. The cycle begins and ends with all prosthetic groups in their oxidized forms (shown in blue). Reduced forms are in red. Four cytochrome c molecules donate four electrons, which, in allowing the binding and cleavage of an O2 molecule, also makes possible the import of four H+ from the matrix to form two molecules of H2O, which are released from the enzyme to regenerate the initial state.
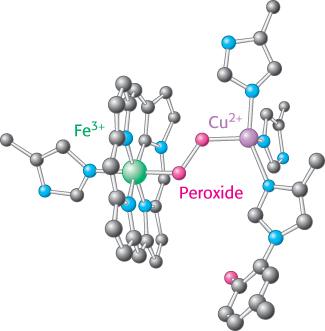
FIGURE 18.15Peroxide bridge. The oxygen bound to heme a3 is reduced to peroxide by the presence of CuB.
Electrons from two molecules of reduced cytochrome c flow down an electron-transfer pathway within cytochrome c oxidase, one stopping at CuB and the other at heme a3. With both centers in the reduced state, they together can now bind an oxygen molecule.
As molecular oxygen binds, it abstracts an electron from each of the nearby ions in the active center to form a peroxide (O22−) bridge between them (Figure 18.15).
Two more molecules of cytochrome c bind and release electrons that travel to the active center. The addition of an electron as well as H+ to each oxygen atom reduces the two ion–oxygen groups to CuB2+—OH and Fe3+—OH.
Reaction with two more H+ ions allows the release of two molecules of H2O and resets the enzyme to its initial, fully oxidized form.
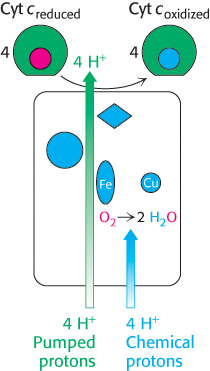
FIGURE 18.16Proton transport by cytochrome c oxidase. Four protons are taken up from the matrix side to reduce one molecule of O2 to two molecules of H2O. These protons are called “chemical protons” because they participate in a clearly defined reaction with O2. Four additional “pumped” protons are transported out of the matrix and released on the cytoplasmic side in the course of the reaction. The pumped protons double the efficiency of free-energy storage in the form of a proton gradient for this final step in the electron-transport chain.
The four protons in this reaction come exclusively from the matrix. Thus, the consumption of these four protons contributes directly to the proton gradient. Recall that each proton contributes 21.8 kJ mol−1 (5.2 kcal mol−1) to the free energy associated with the proton gradient; so these four protons contribute 87.2 kJ mol−1 (20.8 kcal mol−1), an amount substantially less than the free energy available from the reduction of oxygen to water. What is the fate of this missing energy? Remarkably, cytochrome c oxidase uses this energy to pump four additional protons from the matrix to the cytoplasmic side of the membrane in the course of each reaction cycle for a total of eight protons removed from the matrix (Figure 18.16). The details of how these protons are transported through the protein are still under study. However, two effects contribute to the mechanism. First, charge neutrality tends to be maintained in the interior of proteins. Thus, the addition of an electron to a site inside a protein tends to favor the binding of H+ to a nearby site. Second, conformational changes take place, particularly around the heme a3–CuB center, in the course of the reaction cycle. Presumably, in one conformation, protons may enter the protein exclusively from the matrix side, whereas, in another, they may exit exclusively to the cytoplasmic side. Thus, the overall process catalyzed by cytochrome c oxidase is
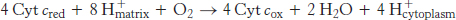
Figure 18.17 summarizes the flow of electrons from NADH and FADH2 through the respiratory chain. This series of exergonic reactions is coupled to the pumping of protons from the matrix. As we will see shortly, the energy inherent in the proton gradient will be used to synthesize ATP.
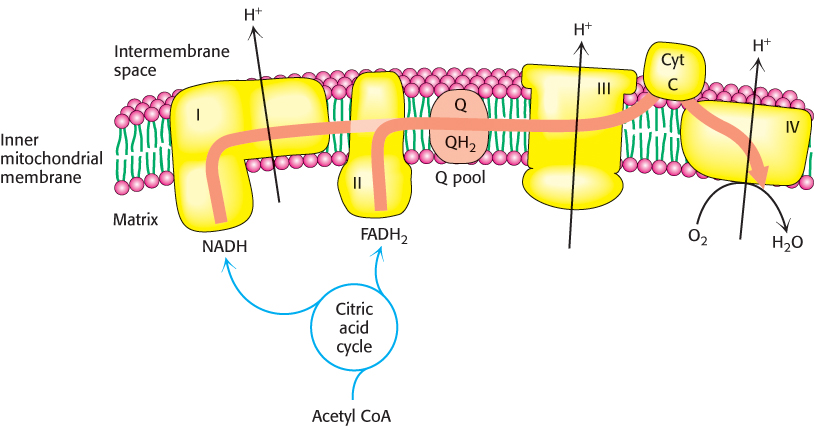
FIGURE 18.17The electron-transport chain. High-energy electrons in the form of NADH and FADH2 are generated by the citric acid cycle. These electrons flow through the respiratory chain, which powers proton pumping and results in the reduction of O2.
Toxic derivatives of molecular oxygen such as superoxide radicals are scavenged by protective enzymes
As discussed earlier, molecular oxygen is an ideal terminal electron acceptor, because its high affinity for electrons provides a large thermodynamic driving force. However, danger lurks in the reduction of O2. The transfer of four electrons leads to safe products (two molecules of H2O), but partial reduction generates hazardous compounds. In particular, the transfer of a single electron to O2 forms superoxide ion, whereas the transfer of two electrons yields peroxide.
Both compounds are potentially destructive. The strategy for the safe reduction of O2 is clear: the catalyst does not release partly reduced intermediates. Cytochrome c oxidase meets this crucial criterion by holding O2 tightly between Fe and Cu ions.
 Although cytochrome c oxidase and other proteins that reduce O2 are remarkably successful in not releasing intermediates, small amounts of superoxide anion and hydrogen peroxide are unavoidably formed. Superoxide, hydrogen peroxide, and species that can be generated from them such as the hydroxyl radical (OH·) are collectively referred to as reactive oxygen species or ROS. Oxidative damage caused by ROS has been implicated in the aging process as well as in a growing list of diseases (Table 18.3).
Although cytochrome c oxidase and other proteins that reduce O2 are remarkably successful in not releasing intermediates, small amounts of superoxide anion and hydrogen peroxide are unavoidably formed. Superoxide, hydrogen peroxide, and species that can be generated from them such as the hydroxyl radical (OH·) are collectively referred to as reactive oxygen species or ROS. Oxidative damage caused by ROS has been implicated in the aging process as well as in a growing list of diseases (Table 18.3).
TABLE 18.3 Pathological conditions that may entail free-radical injury
|
|
|
|
|
|
Duchenne muscular dystrophy |
|
|
|
|
|
|
|
|
|
|
Retrolental fibroplasia (conversion of the retina into a fibrous mass in premature infants) |
Cerebrovascular disorders |
Ischemia; reperfusion injury |
Information from Michael Lieberman and Allan D. Marks, Basic Medical Biochemistry: A Clinical Approach, 4th ed. (Lippincott, Williams & Wilkins, 2012), p. 437. |
Dismutation
A reaction in which a single reactant is converted into two different products.
What are the cellular defense strategies against oxidative damage by ROS? Chief among them is the enzyme superoxide dismutase. This enzyme scavenges superoxide radicals by catalyzing the conversion of two of these radicals into hydrogen peroxide and molecular oxygen.
Eukaryotes contain two forms of this enzyme, a manganese-containing version located in mitochondria and a copper-and-zinc-dependent cytoplasmic form. These enzymes perform the dismutation reaction by a similar mechanism (Figure 18.18). The oxidized form of the enzyme is reduced by superoxide to form oxygen. The reduced form of the enzyme, formed in this reaction, then reacts with a second superoxide ion to form peroxide, which takes up two protons along the reaction path to yield hydrogen peroxide.
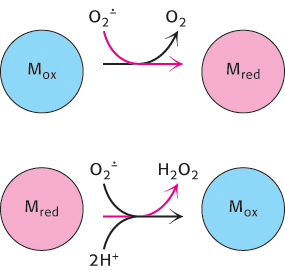
FIGURE 18.18Superoxide dismutase mechanism. The oxidized form of superoxide dismutase (Mox) reacts with one superoxide ion to form O2 and generate the reduced form of the enzyme (Mred). The reduced form then reacts with a second superoxide and two protons to form hydrogen peroxide and regenerate the oxidized form of the enzyme.
The hydrogen peroxide formed by superoxide dismutase and by other processes is scavenged by catalase, a ubiquitous heme protein that catalyzes the dismutation of hydrogen peroxide into water and molecular oxygen.
Superoxide dismutase and catalase are remarkably efficient, performing their reactions at or near the diffusion-limited rate (Section 8.4). Glutathione peroxidase also plays a role in scavenging H2O2 (Section 20.5). Other cellular defenses against oxidative damage include the antioxidant vitamins, vitamins E and C. Because it is lipophilic, vitamin E is especially useful in protecting membranes from lipid peroxidation.
 A long-term benefit of exercise may be to increase the amount of superoxide dismutase in the cell. The elevated aerobic metabolism during exercise causes more ROS to be generated. In response, the cell synthesizes more protective enzymes. The net effect is one of protection, because the increase in superoxide dismutase more effectively protects the cell during periods of rest (Problem 48).
A long-term benefit of exercise may be to increase the amount of superoxide dismutase in the cell. The elevated aerobic metabolism during exercise causes more ROS to be generated. In response, the cell synthesizes more protective enzymes. The net effect is one of protection, because the increase in superoxide dismutase more effectively protects the cell during periods of rest (Problem 48).
Despite the fact that reactive oxygen species are known hazards, recent evidence suggests that, under certain circumstances, the controlled generation of these molecules may be an important component of signal-transduction pathways. For instance, growth factors have been shown to increase ROS levels as part of their signaling pathway, and ROS regulate channels and transcription factors. ROS have been implicated in the control of cell differentiation, the immune response, autophagy as well as other metabolic activities. The dual role of ROS is an excellent example of the wondrous complexity of biochemistry of living systems: even potentially harmful substances can be harnessed to play useful roles.
Electrons can be transferred between groups that are not in contact
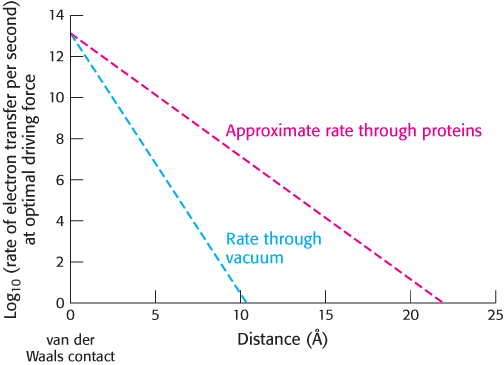
FIGURE 18.19Distance dependence of electron-transfer rate. The rate of electron transfer decreases as the electron donor and the electron acceptor move apart. In a vacuum, the rate decreases by a factor of 10 for every increase of 0.8 Å. In proteins, the rate decreases more gradually, by a factor of 10 for every increase of 1.7 Å. This rate is only approximate because variations in the structure of the intervening protein medium can affect the rate.
How are electrons transferred between electron-carrying groups of the respiratory chain? This question is intriguing because these groups are frequently buried in the interior of a protein in fixed positions and are therefore not directly in contact with one another. Electrons can move through space, even through a vacuum. However, the rate of electron transfer through space falls off rapidly as the electron donor and electron acceptor move apart from each other, decreasing by a factor of 10 for each increase in separation of 0.8 Å. The protein environment provides more-efficient pathways for electron conduction: typically, the rate of electron transfer decreases by a factor of 10 every 1.7 Å (Figure 18.19). For groups in contact, electron-transfer reactions can be quite fast, with rates of approximately 1013 s−1. Within proteins in the electron-transport chain, electron-carrying groups are typically separated by 15 Å beyond their van der Waals contact distance. For such separations, we expect electron-transfer rates of approximately 104 s−1 (i.e., electron transfer in less than 1 ms), assuming that all other factors are optimal. Without the mediation of the protein, an electron transfer over this distance would take approximately 1 day.
The case is more complicated when electrons must be transferred between two distinct proteins, such as when cytochrome c accepts electrons from Complex III or passes them on to Complex IV. A series of hydrophobic interactions bring the heme groups of cytochrome c and c1 to within 4.5 Å of each other, with the iron atoms separated by 17.4 Å. This distance could allow cytochrome c reduction at a rate of 8.3 × 106 s−1.
The conformation of cytochrome c has remained essentially constant for more than a billion years
 Cytochrome c is present in all organisms having mitochondrial respiratory chains: plants, animals, and eukaryotic microorganisms. This electron carrier evolved more than 1.5 billion years ago, before the divergence of plants and animals. Its function has been conserved throughout this period, as evidenced by the fact that the cytochrome c of any eukaryotic species reacts in vitro with the cytochrome c oxidase of any other species tested thus far. For example, wheat-germ cytochrome c reacts with human cytochrome c oxidase. Additionally, some prokaryotic cytochromes, such as cytochrome c2 from the photosynthetic bacterium Rhodospirillum rubrum and cytochrome c550 from the denitrifying bacterium Paracoccus denitrificans, closely resemble cytochrome c from tuna-heart mitochondria (Figure 18.20). This evidence attests to an efficient evolutionary solution to electron transfer bestowed by the structural and functional characteristics of cytochrome c.
Cytochrome c is present in all organisms having mitochondrial respiratory chains: plants, animals, and eukaryotic microorganisms. This electron carrier evolved more than 1.5 billion years ago, before the divergence of plants and animals. Its function has been conserved throughout this period, as evidenced by the fact that the cytochrome c of any eukaryotic species reacts in vitro with the cytochrome c oxidase of any other species tested thus far. For example, wheat-germ cytochrome c reacts with human cytochrome c oxidase. Additionally, some prokaryotic cytochromes, such as cytochrome c2 from the photosynthetic bacterium Rhodospirillum rubrum and cytochrome c550 from the denitrifying bacterium Paracoccus denitrificans, closely resemble cytochrome c from tuna-heart mitochondria (Figure 18.20). This evidence attests to an efficient evolutionary solution to electron transfer bestowed by the structural and functional characteristics of cytochrome c.
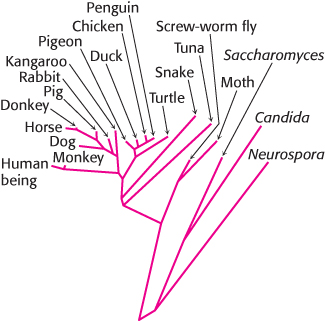
FIGURE 18.21Evolutionary tree constructed from sequences of cytochrome c. Branch lengths are proportional to the number of amino acid changes that are believed to have occurred.
[Information from Walter M. Fitch and Emanuel Margoliash.]
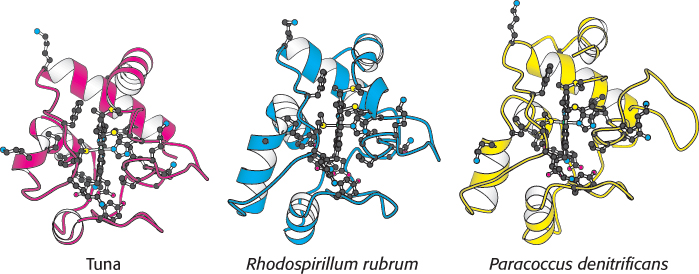
 FIGURE 18.20 Conservation of the three-dimensional structure of cytochrome c. Notice the overall structural similarity of the different molecules from different sources. The side chains are shown for the 21 conserved amino acids as well as the centrally located planar heme.
FIGURE 18.20 Conservation of the three-dimensional structure of cytochrome c. Notice the overall structural similarity of the different molecules from different sources. The side chains are shown for the 21 conserved amino acids as well as the centrally located planar heme.
[Drawn from 3CYT.pdb, 3C2C.pdb, and 155C.pdb.]
The resemblance among cytochrome c molecules extends to the level of amino acid sequence. Because of the molecule’s small size and ubiquity, the amino acid sequences of cytochrome c from more than 80 widely ranging eukaryotic species have been determined by direct protein sequencing. The striking finding is that 21 of 104 residues have been invariant for more than one and a half billion years of evolution. A phylogenetic tree, constructed from the amino acid sequences of cytochrome c, reveals the evolutionary relationships between many animal species (Figure 18.21).



 The importance of Fe-
The importance of Fe-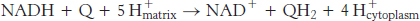



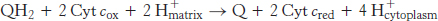

 FIGURE 18.11 Structure of Q-
FIGURE 18.11 Structure of Q-
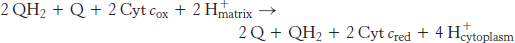
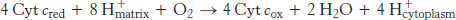

 FIGURE 18.13 Structure of cytochrome c oxidase. This enzyme consists of 13 polypeptide chains. Notice that most of the complex, as well as two major prosthetic groups (heme a and heme a3–CuB) are embedded in the membrane (α helices represented by vertical tubes). Heme a3–CuB is the site of the reduction of oxygen to water. The CuA/CuA prosthetic group is positioned near the intermembrane space to better accept electrons from cytochrome c. CO(bb) is a carbonyl group of the peptide backbone.
FIGURE 18.13 Structure of cytochrome c oxidase. This enzyme consists of 13 polypeptide chains. Notice that most of the complex, as well as two major prosthetic groups (heme a and heme a3–CuB) are embedded in the membrane (α helices represented by vertical tubes). Heme a3–CuB is the site of the reduction of oxygen to water. The CuA/CuA prosthetic group is positioned near the intermembrane space to better accept electrons from cytochrome c. CO(bb) is a carbonyl group of the peptide backbone.



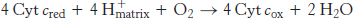

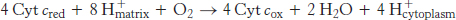

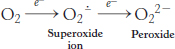
 Although cytochrome c oxidase and other proteins that reduce O2 are remarkably successful in not releasing intermediates, small amounts of superoxide anion and hydrogen peroxide are unavoidably formed. Superoxide, hydrogen peroxide, and species that can be generated from them such as the hydroxyl radical (OH·) are collectively referred to as reactive oxygen species or ROS. Oxidative damage caused by ROS has been implicated in the aging process as well as in a growing list of diseases (Table 18.3).
Although cytochrome c oxidase and other proteins that reduce O2 are remarkably successful in not releasing intermediates, small amounts of superoxide anion and hydrogen peroxide are unavoidably formed. Superoxide, hydrogen peroxide, and species that can be generated from them such as the hydroxyl radical (OH·) are collectively referred to as reactive oxygen species or ROS. Oxidative damage caused by ROS has been implicated in the aging process as well as in a growing list of diseases (Table 18.3).


 A long-
A long-
 Cytochrome c is present in all organisms having mitochondrial respiratory chains: plants, animals, and eukaryotic microorganisms. This electron carrier evolved more than 1.5 billion years ago, before the divergence of plants and animals. Its function has been conserved throughout this period, as evidenced by the fact that the cytochrome c of any eukaryotic species reacts in vitro with the cytochrome c oxidase of any other species tested thus far. For example, wheat-
Cytochrome c is present in all organisms having mitochondrial respiratory chains: plants, animals, and eukaryotic microorganisms. This electron carrier evolved more than 1.5 billion years ago, before the divergence of plants and animals. Its function has been conserved throughout this period, as evidenced by the fact that the cytochrome c of any eukaryotic species reacts in vitro with the cytochrome c oxidase of any other species tested thus far. For example, wheat-

 FIGURE 18.20 Conservation of the three-
FIGURE 18.20 Conservation of the three-