28.5DNA Recombination Plays Important Roles in Replication, Repair, and Other Processes
DNA Recombination Plays Important Roles in Replication, Repair, and Other Processes
Most processes associated with DNA replication function to copy the genetic message as faithfully as possible. However, several biochemical processes require the recombination of genetic material between two DNA molecules. In genetic recombination, two daughter molecules are formed by the exchange of genetic material between two parent molecules (Figure 28.43). Recombination is essential in the following processes.

853
1. When replication stalls, recombination processes can reset the replication machinery so that replication can continue.
2. Some double-
3. In meiosis, the limited exchange of genetic material between paired chromosomes provides a simple mechanism for generating genetic diversity in a population.
4. As we shall see in Chapter 34, recombination plays a crucial role in generating molecular diversity for antibodies and some other molecules in the immune system.
5. Some viruses employ recombination pathways to integrate their genetic material into the DNA of a host cell.
6. Recombination is used to manipulate genes in, for example, the generation of “gene knockout” mice and other targeted genome modifications (Section 5.4).
Recombination is most efficient between DNA sequences that are similar in sequence. In homologous recombination, parent DNA duplexes align at regions of sequence similarity, and new DNA molecules are formed by the breakage and joining of homologous segments.
RecA can initiate recombination by promoting strand invasion
In many recombination pathways, a DNA molecule with a free end recombines with a DNA molecule having no free ends available for interaction. DNA molecules with free ends are the common result of double-

854
Some recombination reactions proceed through Holliday-junction intermediates
In recombination pathways for meiosis and some other processes, intermediates form that are composed of four polynucleotide chains in a crosslike structure. Intermediates with these crosslike structures are often referred to as Holliday junctions, after Robin Holliday, who proposed their role in recombination in 1964. Such intermediates have been characterized by a wide range of techniques, including x-
Specific enzymes, termed recombinases, bind to these structures and resolve them into separated DNA duplexes. The Cre recombinase from bacteriophage P1 has been extensively studied. The mechanism begins with the recombinase binding to the DNA substrates (Figure 28.45).
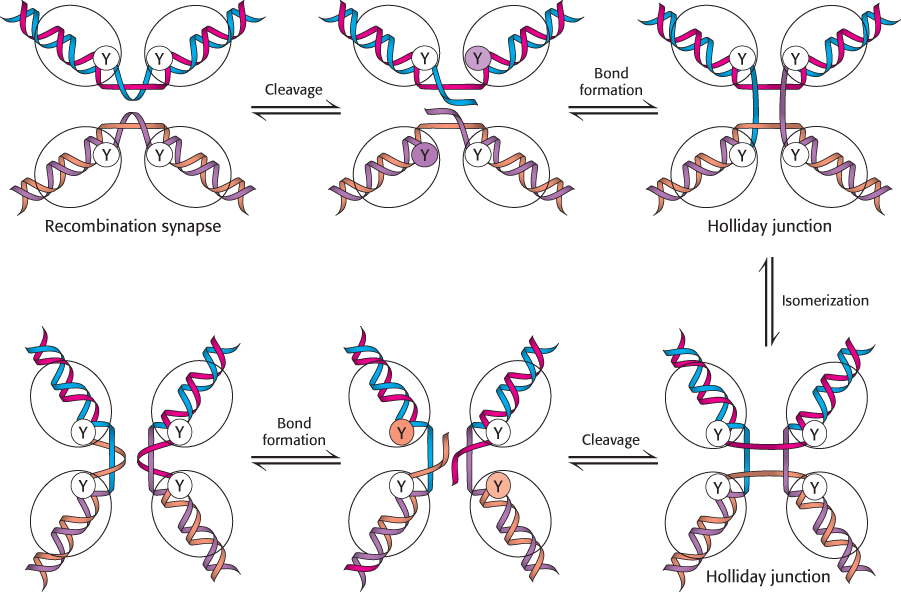
Four molecules of the enzyme and two DNA molecules come together to form a recombination synapse. The reaction begins with the cleavage of one strand from each duplex via transesterification reactions with the 3′-phosphoryl groups becoming linked to specific tyrosine residues in the recombinase while the 5′-hydroxyl group of each cleaved strand remains free. The free 5′ ends invade the other duplex and participate in transesterification reactions to form new phosphodiester bonds and free the tyrosine residues. These reactions result in the formation of a Holliday junction. This junction can then isomerize to form a structure in which the polynucleotide chains in the center of the structure are reoriented. From this junction, the processes of strand cleavage and phosphodiester-
Cre catalyzes the formation of Holliday junctions as well as their resolution. In contrast, other proteins bind to Holliday junctions that have already been formed by other processes and resolve them into separate duplexes. In many cases, these proteins also promote the process of branch migration whereby a Holliday junction is moved along the two component double helices. Branch migration can affect which segments of DNA are exchanged in a recombination process.
855
 Recombination can also occur during cell division between different, non-
Recombination can also occur during cell division between different, non-