7.1Myoglobin and Hemoglobin Bind Oxygen at Iron Atoms in Heme
Myoglobin and Hemoglobin Bind Oxygen at Iron Atoms in Heme
Sperm whale myoglobin was the first protein for which the three-
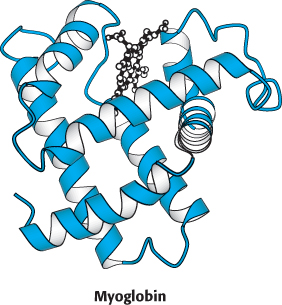
 FIGURE 7.1 Structure of myoglobin. Notice that myoglobin consists of a single polypeptide chain, formed of α helices connected by turns, with one oxygen-
FIGURE 7.1 Structure of myoglobin. Notice that myoglobin consists of a single polypeptide chain, formed of α helices connected by turns, with one oxygen-Myoglobin can exist in an oxygen-
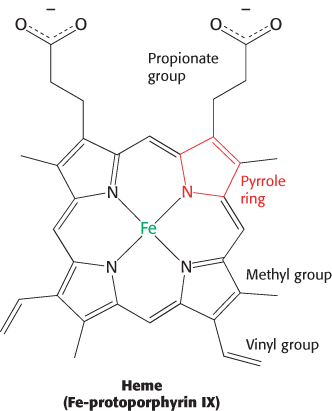
The heme group gives muscle and blood their distinctive red color. It consists of an organic component and a central iron atom. The organic component, called protoporphyrin, is made up of four pyrrole rings linked by methine bridges to form a tetrapyrrole ring. Four methyl groups, two vinyl groups, and two propionate side chains are attached to the central tetrapyrrole.
193
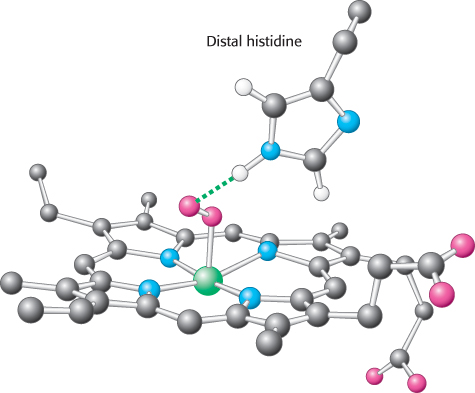
The iron atom lies in the center of the protoporphyrin, bonded to the four pyrrole nitrogen atoms. Although the heme-
Oxygen binding occurs at the sixth coordination site. In deoxymyoglobin, this site remains unoccupied. The iron ion is slightly too large to fit into the well-
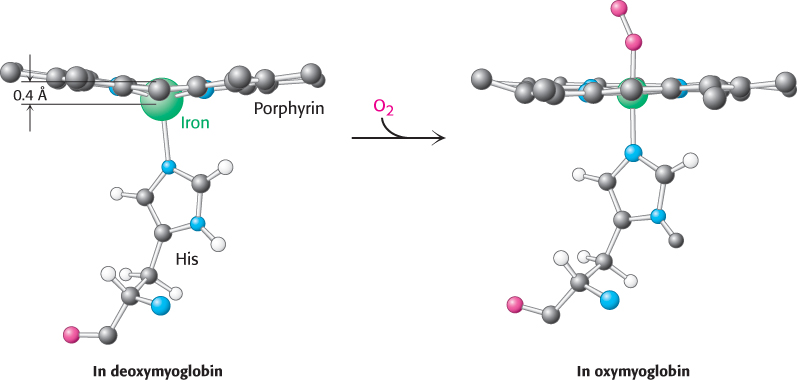
Changes in heme electronic structure upon oxygen binding are the basis for functional imaging studies
 The change in electronic structure that occurs when the iron ion moves into the plane of the porphyrin is paralleled by alterations in the magnetic properties of hemoglobin; these changes are the basis for functional magnetic resonance imaging (fMRI), one of the most powerful methods for examining brain function. Nuclear magnetic resonance techniques detect signals that originate primarily from the protons in water molecules and are altered by the magnetic properties of hemoglobin. With the use of appropriate techniques, images can be generated that reveal differences in the relative amounts of deoxy-
The change in electronic structure that occurs when the iron ion moves into the plane of the porphyrin is paralleled by alterations in the magnetic properties of hemoglobin; these changes are the basis for functional magnetic resonance imaging (fMRI), one of the most powerful methods for examining brain function. Nuclear magnetic resonance techniques detect signals that originate primarily from the protons in water molecules and are altered by the magnetic properties of hemoglobin. With the use of appropriate techniques, images can be generated that reveal differences in the relative amounts of deoxy-
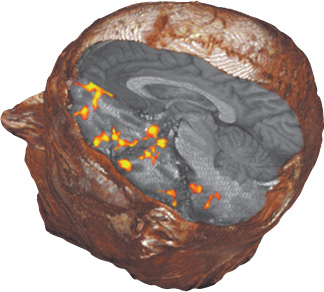
These noninvasive methods identify areas of the brain that process sensory information. For example, subjects have been imaged while breathing air that either does or does not contain odorants. When odorants are present, fMRI detects an increase in the level of hemoglobin oxygenation (and, hence, of activity) in several regions of the brain (Figure 7.3). These regions are in the primary olfactory cortex, as well as in areas in which secondary processing of olfactory signals presumably takes place. Further analysis reveals the time course of activation of particular regions. Functional MRI shows tremendous potential for mapping regions and pathways engaged in processing sensory information obtained from all the senses. A seemingly incidental aspect of the biochemistry of hemoglobin has enabled observation of the brain in action.
194
The structure of myoglobin prevents the release of reactive oxygen species
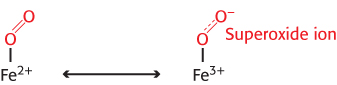
Oxygen binding to iron in heme is accompanied by the partial transfer of an electron from the ferrous ion to oxygen. In many ways, the structure is best described as a complex between ferric ion (Fe3+) and superoxide anion  , as illustrated in Figure 7.4. It is crucial that oxygen, when it is released, leaves as dioxygen rather than superoxide, for two important reasons. First, superoxide and other species generated from it are reactive oxygen species that can be damaging to many biological materials. Second, release of superoxide would leave the iron ion in the ferric state. This species, termed metmyoglobin, does not bind oxygen. Thus, potential oxygen-
, as illustrated in Figure 7.4. It is crucial that oxygen, when it is released, leaves as dioxygen rather than superoxide, for two important reasons. First, superoxide and other species generated from it are reactive oxygen species that can be damaging to many biological materials. Second, release of superoxide would leave the iron ion in the ferric state. This species, termed metmyoglobin, does not bind oxygen. Thus, potential oxygen-
195
Human hemoglobin is an assembly of four myoglobin-like subunits
The three-
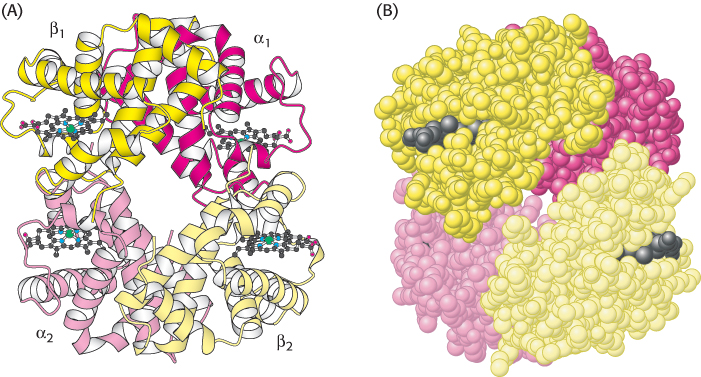
 FIGURE 7.6 Quaternary structure of deoxyhemoglobin. Hemoglobin, which is composed of two α chains and two β chains, functions as a pair of αβ dimers. (A) A ribbon diagram. (B) A space-
FIGURE 7.6 Quaternary structure of deoxyhemoglobin. Hemoglobin, which is composed of two α chains and two β chains, functions as a pair of αβ dimers. (A) A ribbon diagram. (B) A space-The hemoglobin tetramer, referred to as hemoglobin A (HbA), is best described as a pair of identical αβ dimers (α1β1 and α2β2) that associate to form the tetramer. In deoxyhemoglobin, these αβ dimers are linked by an extensive interface, which includes the carboxyl terminus of each chain. The heme groups are well separated in the tetramer by iron–