16.3Glucose Can Be Synthesized from Noncarbohydrate Precursors
Glucose Can Be Synthesized from Noncarbohydrate Precursors
We now turn to the synthesis of glucose from noncarbohydrate precursors, a process called gluconeogenesis. Maintaining levels of glucose is important because the brain depends on glucose as its primary fuel and red blood cells use glucose as their only fuel. The daily glucose requirement of the brain in a typical adult human being is about 120 g, which accounts for most of the 160 g of glucose needed daily by the whole body. The amount of glucose present in body fluids is about 20 g, and that readily available from glycogen is approximately 190 g. Thus, the direct glucose reserves are sufficient to meet glucose needs for about a day. Gluconeogenesis is especially important during a longer period of fasting or starvation (Section 27.5).
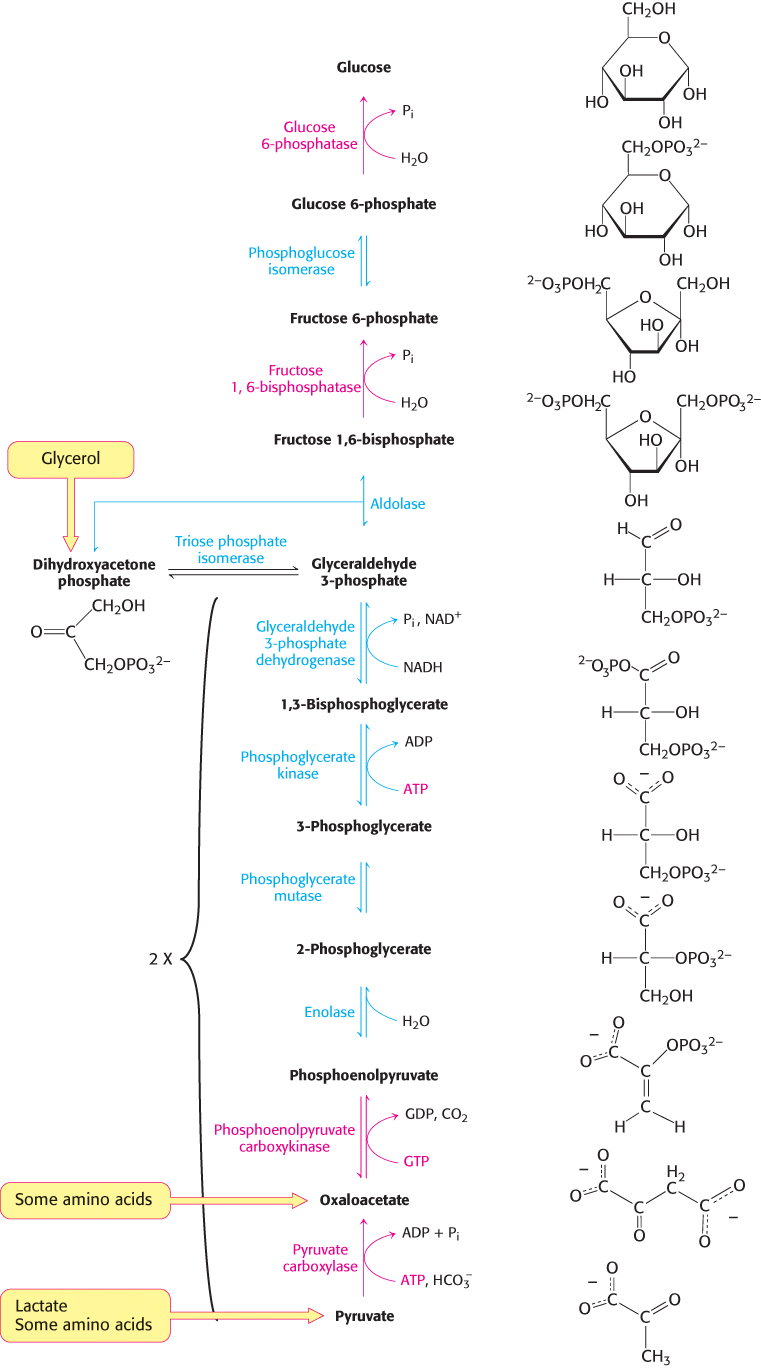
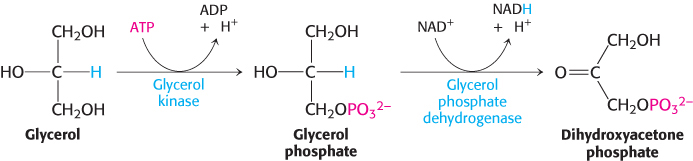
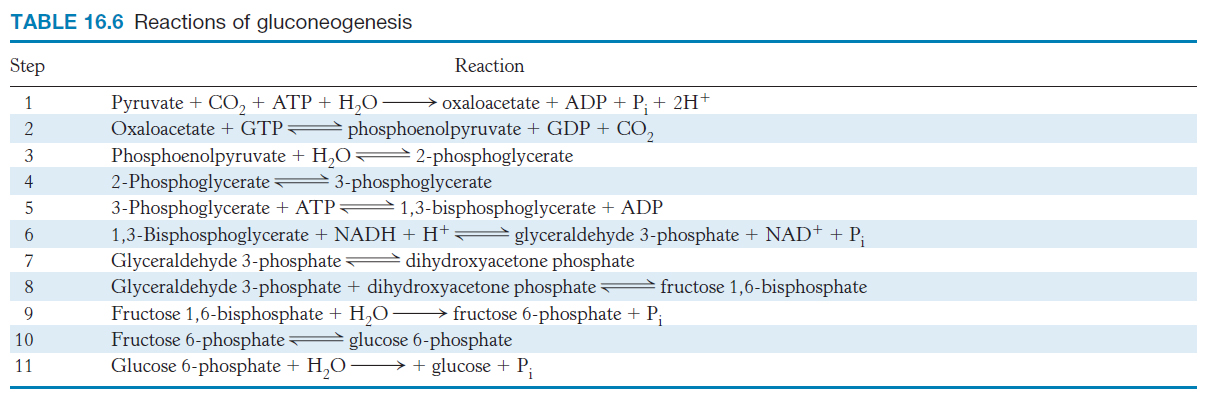
The gluconeogenic pathway converts pyruvate into glucose. Noncarbohydrate precursors of glucose are first converted into pyruvate or enter the pathway at later intermediates such as oxaloacetate and dihydroxyacetone phosphate (Figure 16.24). The major noncarbohydrate precursors are lactate, amino acids, and glycerol. Lactate is formed by active skeletal muscle when the rate of glycolysis exceeds the rate of oxidative metabolism. Lactate is readily converted into pyruvate by the action of lactate dehydrogenase. Amino acids are derived from proteins in the diet and, during starvation, from the breakdown of proteins in skeletal muscle (Section 23.1). The hydrolysis of triacylglycerols (Section 22.2) in fat cells yields glycerol and fatty acids. Glycerol is a precursor of glucose, but animals cannot convert fatty acids into glucose, for reasons that will be given later. Glycerol may enter either the gluconeogenic or the glycolytic pathway at dihydroxyacetone phosphate.
477
478
The major site of gluconeogenesis is the liver, with a small amount also taking place in the kidney. Little gluconeogenesis takes place in the brain, skeletal muscle, or heart muscle. Rather, gluconeogenesis in the liver and kidney helps to maintain the glucose level in the blood so that the brain and muscle can extract sufficient glucose from it to meet their metabolic demands.
Gluconeogenesis is not a reversal of glycolysis
In glycolysis, glucose is converted into pyruvate; in gluconeogenesis, pyruvate is converted into glucose. However, gluconeogenesis is not a reversal of glycolysis. Several reactions must differ because the equilibrium of glycolysis lies far on the side of pyruvate formation. The actual free energy change for the formation of pyruvate from glucose is about −90 kJ mol−1 (−22 kcal mol−1) under typical cellular conditions. Most of the decrease in free energy in glycolysis takes place in the three essentially irreversible steps catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase.
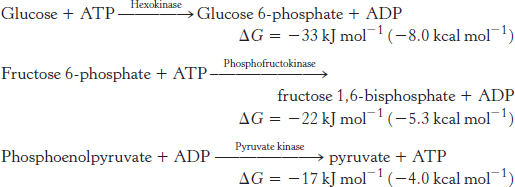
In gluconeogenesis, these virtually irreversible reactions of glycolysis must be bypassed.
The conversion of pyruvate into phosphoenolpyruvate begins with the formation of oxaloacetate
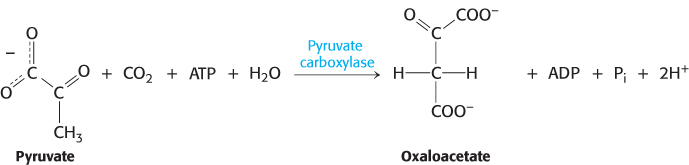
The first step in gluconeogenesis is the carboxylation of pyruvate to form oxaloacetate at the expense of a molecule of ATP, a reaction catalyzed by pyruvate carboxylase. This reaction occurs in the mitochondria.
479
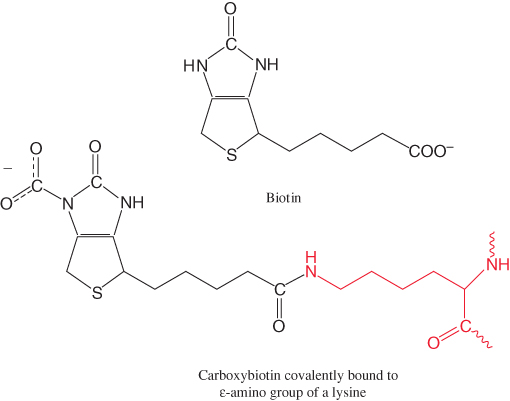
Pyruvate carboxylase requires biotin, a covalently attached prosthetic group, which serves as the carrier of activated CO2. The carboxylate group of biotin is linked to the ε-amino group of a specific lysine residue by an amide bond (Figure 16.25). Recall that, in aqueous solutions, CO2 exists primarily as 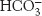 with the aid of carbonic anhydrase (Section 9.2).
with the aid of carbonic anhydrase (Section 9.2).
The carboxylation of pyruvate takes place in three stages:
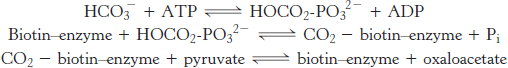
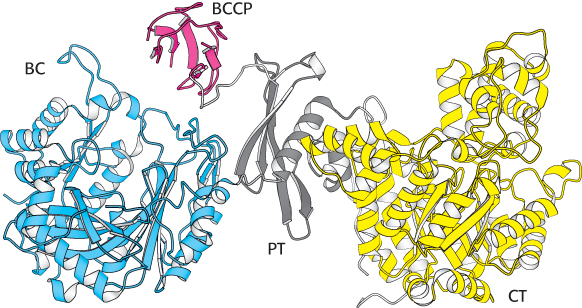
Pyruvate carboxylase functions as a tetramer composed of four identical subunits, and each subunit consists of four domains (Figure 16.26). The biotin carboxylase domain (BC) catalyzes the formation of carboxyphosphate and the subsequent attachment of CO2 to the second domain, the biotin carboxyl carrier protein (BCCP), the site of the covalently attached biotin. Once bound to CO2, BCCP leaves the biotin carboxylase active site and swings almost the entire length of the subunit (≈75Å) to the active site of the pyruvate carboxylase domain (PC), which transfers the CO2 to pyruvate to form oxaloacetate. BCCP in one subunit interacts with the active sites on an adjacent subunit. The fourth domain (PT) facilitates the formation of the tetramer.
480
The first partial reaction of pyruvate carboxylase, the formation of carboxybiotin, depends on the presence of acetyl CoA. Biotin is not carboxylated unless acetyl CoA is bound to the enzyme. Acetyl CoA has no effect on the second partial reaction. The allosteric activation of pyruvate carboxylase by acetyl CoA is an important physiological control mechanism that will be discussed in Section 17.4.
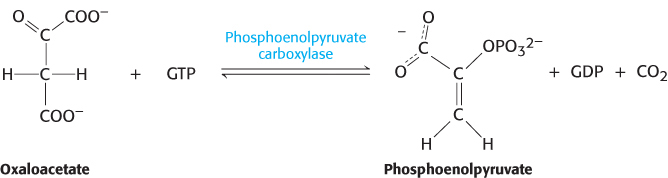
Oxaloacetate is shuttled into the cytoplasm and converted into phosphoenolpyruvate
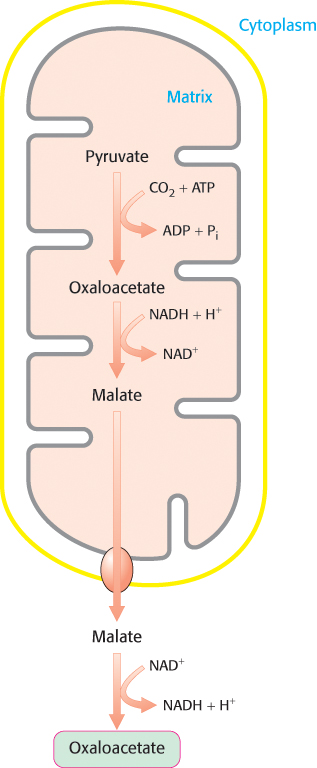
Oxaloacetate must thus be transported to the cytoplasm to complete the synthesis of phosphoenolpyruvate. Oxaloacetate is first reduced to malate by malate dehydrogenase. Malate is transported across the mitochondrial membrane and reoxidized to oxaloacetate by a cytoplasmic NAD+-linked malate dehydrogenase (Figure 16.27). The formation of oxaloacetate from malate also provides NADH for use in subsequent steps in gluconeogenesis. Finally, oxaloacetate is simultaneously decarboxylated and phosphorylated by phosphoenolpyruvate carboxykinase (PEPCK) to generate phosphoenolpyruvate. The phosphoryl donor is GTP. The CO2 that was added to pyruvate by pyruvate carboxylase comes off in this step.
The sum of the reactions catalyzed by pyruvate carboxylase and phosphoenolpyruvate carboxykinase is
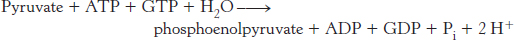
This pair of reactions bypasses the irreversible reaction catalyzed by pyruvate kinase in glycolysis.
Why is a carboxylation and a decarboxylation required to form phosphoenolpyruvate from pyruvate? Recall that, in glycolysis, the presence of a phosphoryl group traps the unstable enol isomer of pyruvate as phosphoenolpyruvate. However, the addition of a phosphoryl group to pyruvate is a highly unfavorable reaction: the ΔG°′ of the reverse of the glycolytic reaction catalyzed by pyruvate kinase is +31 kJ mol−1 (+7.5 kcal mol−1). In gluconeogenesis, the use of the carboxylation and decarboxylation steps results in a much more favorable ΔG°′. The formation of phosphoenolpyruvate from pyruvate in the gluconeogenic pathway has a ΔG°′ of +0.8 kJ mol−1 (+0.23kcal mol−1). A molecule of ATP is used to power the addition of a molecule of CO2 to pyruvate in the carboxylation step. That CO2 is then removed to power the formation of phosphoenolpyruvate in the decarboxylation step. Decarboxylations often drive reactions that are otherwise highly endergonic. This metabolic motif is used in the citric acid cycle (Chapter 17), the pentose phosphate pathway (Chapter 20), and fatty acid synthesis (Section 22.4).
The conversion of fructose 1,6-bisphosphate into fructose 6-phosphate and orthophosphate is an irreversible step
On formation, phosphoenolpyruvate is metabolized by the enzymes of glycolysis but in the reverse direction. These reactions are near equilibrium under intracellular conditions; so, when conditions favor gluconeogenesis, the reverse reactions will take place until the next irreversible step is reached. This step is the hydrolysis of fructose 1,6-
481
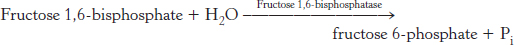
The enzyme responsible for this step is fructose 1,6-
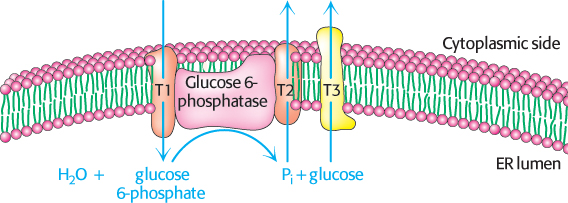
The generation of free glucose is an important control point
The fructose 6-
This final step in the generation of glucose does not take place in the cytoplasm. Rather, glucose 6-
Six high-transfer-potential phosphoryl groups are spent in synthesizing glucose from pyruvate
The formation of glucose from pyruvate is energetically unfavorable unless it is coupled to reactions that are favorable. Compare the stoichiometry of gluconeogenesis with that of the reverse of glycolysis.
The stoichiometry of gluconeogenesis is
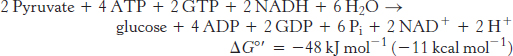
In contrast, the stoichiometry for the reversal of glycolysis is
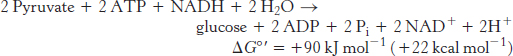
Note that six nucleoside triphosphate molecules are hydrolyzed to synthesize glucose from pyruvate in gluconeogenesis, whereas only two molecules of ATP are generated in glycolysis in the conversion of glucose into pyruvate. Thus, the extra cost of gluconeogenesis is four high-
482