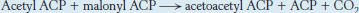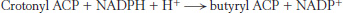22.4Fatty Acids Are Synthesized by Fatty Acid Synthase
Fatty Acids Are Synthesized by Fatty Acid Synthase
Fatty acids are synthesized by a complex of enzymes that together are called fatty acid synthase. Because eating a typical Western diet meets our physiological needs for fats and lipids, adult human beings have little need for de novo fatty acid synthesis. However, many tissues, such as liver and adipose tissue, are capable of synthesizing fatty acids, and this synthesis is required under certain physiological conditions. For instance, fatty acid synthesis is necessary during embryonic development and during lactation in mammary glands. Inappropriate fatty acid synthesis in the liver of alcoholics contributes to liver failure.
Acetyl CoA, the end product of fatty acid degradation, is the precursor for virtually all fatty acids. The biochemical challenge is to link the two carbon units together and reduce the carbons to produce palmitate, a C16 fatty acid. Palmitate then serves as a precursor for the variety of other fatty acids.
Fatty acids are synthesized and degraded by different pathways
Although fatty acid synthesis is the reversal of the degradative pathway in regard to basic chemical reactions, the synthetic and degradative pathways are different mechanistically, again exemplifying the principle that synthetic and degradative pathways are almost always distinct. Some important differences between the pathways are as follows:
Synthesis takes place in the cytoplasm, in contrast with degradation, which takes place primarily in the mitochondrial matrix.
Intermediates in fatty acid synthesis are covalently linked to the sulfhydryl groups of an acyl carrier protein (ACP), whereas intermediates in fatty acid breakdown are covalently attached to the sulfhydryl group of coenzyme A.
The enzymes of fatty acid synthesis in higher organisms are joined in a single polypeptide chain called fatty acid synthase. In contrast, the degradative enzymes are not linked covalently.
The growing fatty acid chain is elongated by the sequential addition of two-
carbon units derived from acetyl CoA. The activated donor of two-carbon units in the elongation step is malonyl ACP. The elongation reaction is driven by the release of CO2. 662
The reductant in fatty acid synthesis is NADPH, whereas the oxidants in fatty acid degradation are NAD+ and FAD.
The isomeric form of the hydroxyacyl intermediate in degradation is l, while the d form is used in synthesis.
The formation of malonyl CoA is the committed step in fatty acid synthesis
Fatty acid synthesis starts with the carboxylation of acetyl CoA to malonyl CoA. This irreversible reaction is the committed step in fatty acid synthesis.
The synthesis of malonyl CoA is catalyzed by the cytoplasmic enzyme acetyl CoA carboxylase 1, which contains a biotin prosthetic group. The carboxyl group of biotin is covalently attached to the ϵ amino group of a lysine residue, as in pyruvate carboxylase (Figure 16.24) and propionyl CoA carboxylase. As with these other enzymes, a carboxybiotin intermediate is formed at the expense of the hydrolysis of a molecule of ATP. The activated CO2 group in this intermediate is then transferred to acetyl CoA to form malonyl CoA.
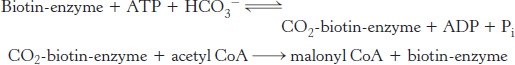
Acetyl CoA carboxylase 2, an isozyme of carboxylase 1 located in the mitochondria, is the essential regulatory enzyme for fatty acid metabolism (Section 22.5).
Intermediates in fatty acid synthesis are attached to an acyl carrier protein
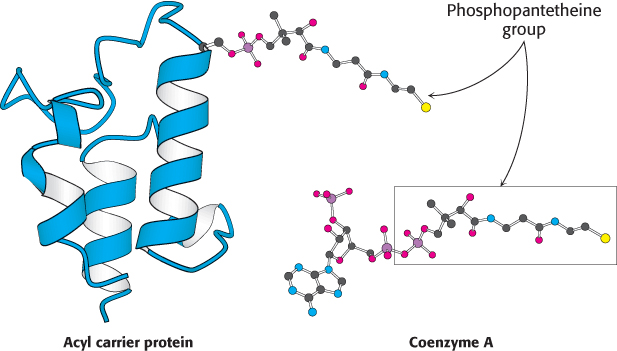
The intermediates in fatty acid synthesis are linked to an acyl carrier protein. Specifically, they are linked to the sulfhydryl terminus of a phosphopantetheine group. In the degradation of fatty acids, this unit is present as part of coenzyme A, whereas, in their synthesis, it is attached to a serine residue of the acyl carrier protein (Figure 22.25). Thus, ACP, a single polypeptide chain of 77 residues, can be regarded as a giant prosthetic group, a “macro CoA.”
Fatty acid synthesis consists of a series of condensation, reduction, dehydration, and reduction reactions
The enzyme system that catalyzes the synthesis of saturated long-
|
Step |
Reaction |
Enzyme |
|---|---|---|
|
1 |
|
Acetyl CoA carboxylase |
|
2 |
|
Acetyl transacylase |
|
3 |
|
Malonyl transacylase |
|
4 |
|
β-Ketoacyl synthase |
|
5 |
|
β-Ketoacyl reductase |
|
6 |
|
3- |
|
7 |
|
Enoyl reductase |
663
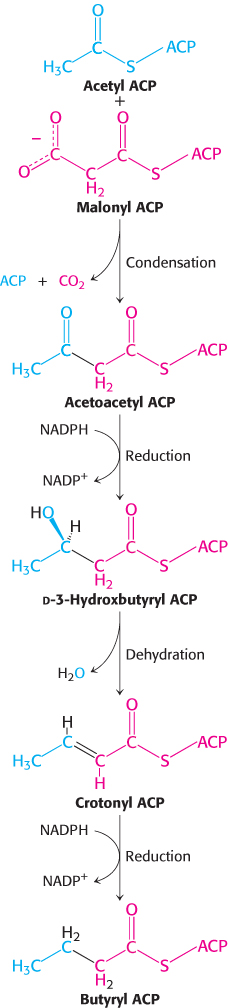
The elongation phase of fatty acid synthesis starts with the formation of acetyl ACP and malonyl ACP. Acetyl transacylase and malonyl transacylase catalyze these reactions.
Malonyl transacylase is highly specific, whereas acetyl transacylase can transfer acyl groups other than the acetyl unit, though at a much slower rate. The synthesis of fatty acids with an odd number of carbon atoms starts with propionyl ACP, which is formed from propionyl CoA by acetyl transacylase.
Acetyl ACP and malonyl ACP react to form acetoacetyl ACP (Figure 22.26). The β-ketoacyl synthase, also called the condensing enzyme, catalyzes this condensation reaction.
In the condensation reaction, a four-
The next three steps in fatty acid synthesis reduce the keto group at C-
664
In the second round of fatty acid synthesis, butyryl ACP condenses with malonyl ACP to form a C6-β-ketoacyl ACP. This reaction is like the one in the first round, in which acetyl ACP condenses with malonyl ACP to form a C4-β-ketoacyl ACP. Reduction, dehydration, and a second reduction convert the C6-β-ketoacyl ACP into a C6-acyl ACP, which is ready for a third round of elongation. The elongation cycles continue until C16-acyl ACP is formed. This intermediate is a good substrate for a thioesterase that hydrolyzes C16-acyl ACP to yield palmitate and ACP. The thioesterase acts as a ruler to determine fatty acid chain length. The synthesis of longer-
Fatty acids are synthesized by a multifunctional enzyme complex in animals
Although the basic biochemical reactions in fatty acid synthesis are very similar in E. coli and eukaryotes, the structure of the synthase varies considerably. The component enzymes of animal fatty acid synthases, in contrast with those of E. coli and plants, are linked in a large polypeptide chain.
The structure of a large part of the mammalian fatty acid synthase has recently been determined, with the acyl carrier protein and thioesterase remaining to be resolved. The enzyme is a dimer of identical 270-
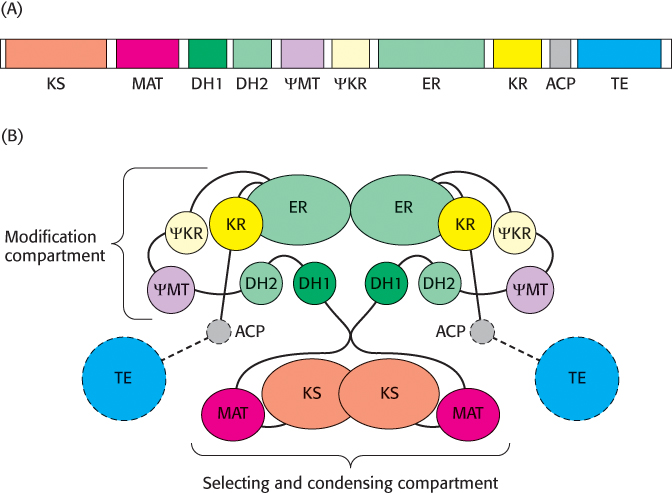
The two component chains interact such that the enzyme activities are partitioned into two distinct compartments (Figure 22.27B). The selecting and condensing compartment binds the acetyl and malonyl substrates and condenses them to form the growing chain. Interestingly, the mammalian fatty acid synthase has one active site, malonyl/acetyl transacylase, that adds both acetyl CoA and malonyl CoA. In contrast, most other fatty acid synthases have two separate enzyme activities, one for acetyl CoA and one for malonyl CoA. The modification compartment is responsible for the reduction and dehydration activities that result in the saturated fatty acid product.
Let us consider one catalytic cycle of the fatty acid synthase complex (Figure 22.28). An elongation cycle begins when malonyl/acetyl transacylase (MAT) moves an acetyl unit from coenzyme A to the acyl carrier protein (ACP). β-Ketosynthase (β-KS) accepts the acetyl unit, which forms a thioester with a cysteine residue at the β-KS active site. The vacant ACP is reloaded by MAT, this time with a malonyl moiety. Malonyl ACP visits the active site of β-KS where the condensation of the two 2-
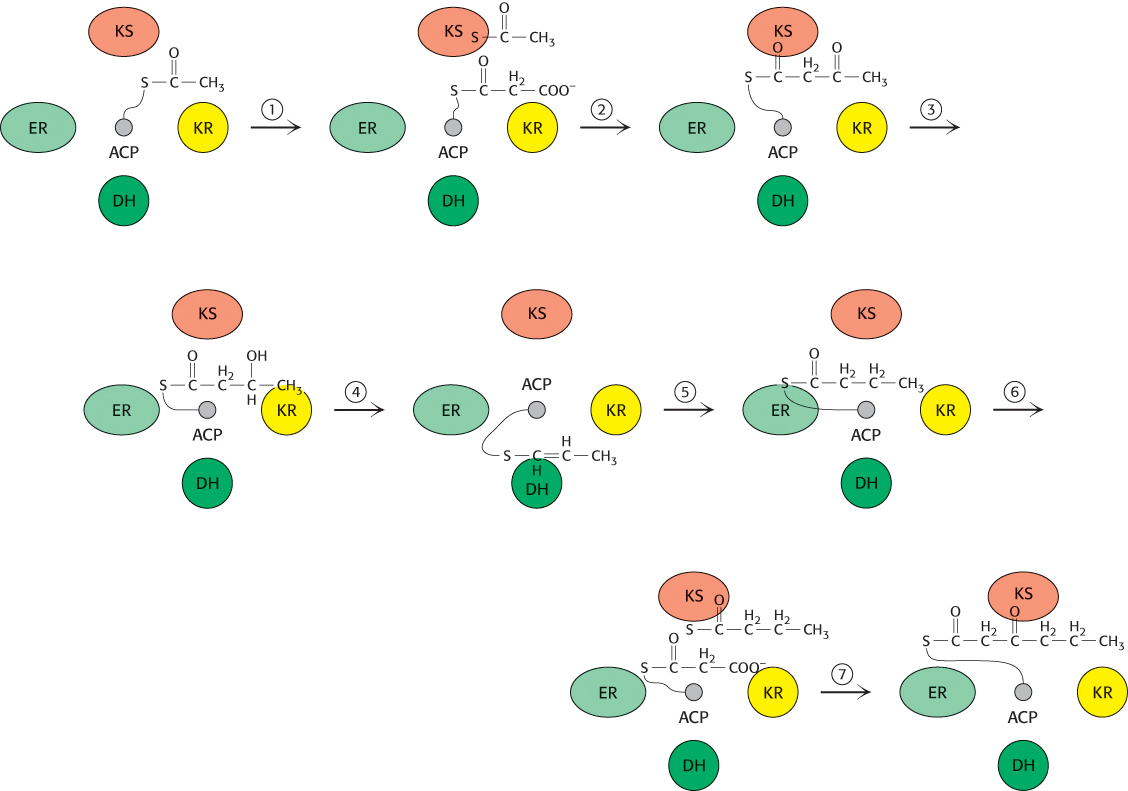
The loaded ACP then sequentially visits the active sites of the modification compartment of the enzyme, where the β-keto group of the substrate is reduced to —OH, dehydrated, and finally reduced to yield the saturated acyl product, still attached to the ACP. With the completion of the modification process, the reduced product is transferred to the β-KS while the ACP accepts another malonyl unit. Condensation takes place and is followed by another modification cycle. The process is repeated until the thioesterase releases the final C16 palmitic acid product.
665
666
Many eukaryotic multienzyme complexes are multifunctional proteins in which different enzymes are linked covalently. Multifunctional enzymes such as fatty acid synthase seem likely to have arisen in eukaryotic evolution by fusion of the individual genes of evolutionary ancestors.
The synthesis of palmitate requires 8 molecules of acetyl CoA, 14 molecules of NADPH, and 7 molecules of ATP
The stoichiometry of the synthesis of palmitate is
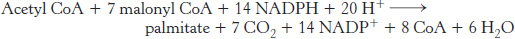
The equation for the synthesis of the malonyl CoA used in the preceding reaction is
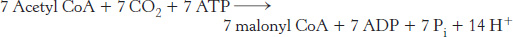
Hence, the overall stoichiometry for the synthesis of palmitate is
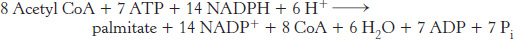
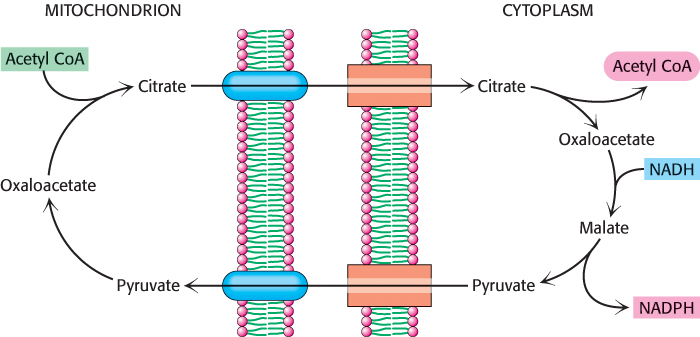
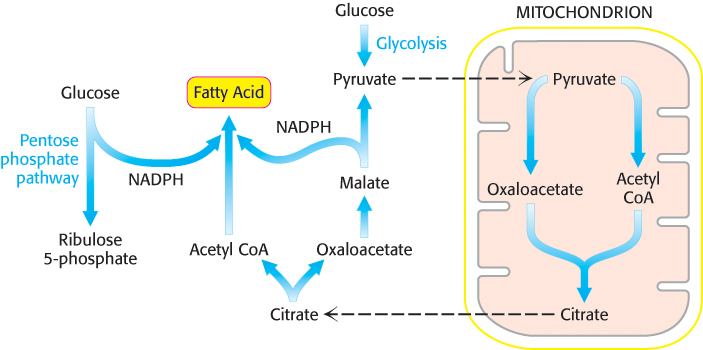
Citrate carries acetyl groups from mitochondria to the cytoplasm for fatty acid synthesis
Fatty acids are synthesized in the cytoplasm, whereas acetyl CoA is formed from pyruvate in mitochondria. Hence, acetyl CoA must be transferred from mitochondria to the cytoplasm for fatty acid synthesis. Mitochondria, however, are not readily permeable to acetyl CoA. Recall that carnitine carries only long-
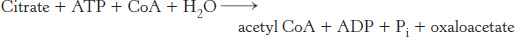
Lyases
Enzymes catalyzing the cleavage of C—
This reaction occurs in three steps: (1) The formation of a phosphoenzyme with the donation of a phosphoryl group from ATP; (2) binding of citrate and CoA followed by the formation of citroyl CoA and release of the phosphate; (3) cleavage of citroyl CoA to yield acetyl CoA and oxaloacetate. As we will see shortly (Section 22.6), citrate stimulates acetyl CoA carboxylase, the enzyme that regulates fatty acid metabolism. Recall also that the presence of citrate in the cytoplasm inhibits phosphofructokinase, the enzyme that controls the glycolytic pathway.
667
ATP-
Several sources supply NADPH for fatty acid synthesis
Oxaloacetate formed in the transfer of acetyl groups to the cytoplasm must now be returned to the mitochondria. The inner mitochondrial membrane is impermeable to oxaloacetate. Hence, a series of bypass reactions are needed. These reactions also generate much of the NADPH needed for fatty acid synthesis. First, oxaloacetate is reduced to malate by NADH. This reaction is catalyzed by a malate dehydrogenase in the cytoplasm.
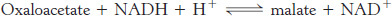
Second, malate is oxidatively decarboxylated by an NADP+-linked malate enzyme (also called malic enzyme).
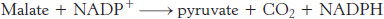
The pyruvate formed in this reaction readily enters mitochondria, where it is carboxylated to oxaloacetate by pyruvate carboxylase.

The sum of these three reactions is
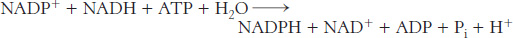
Thus, one molecule of NADPH is generated for each molecule of acetyl CoA that is transferred from mitochondria to the cytoplasm. Hence, eight molecules of NADPH are formed when eight molecules of acetyl CoA are transferred to the cytoplasm for the synthesis of palmitate. The additional six molecules of NADPH required for this process come from the pentose phosphate pathway (Section 20.3).
The accumulation of the precursors for fatty acid synthesis is a wonderful example of the coordinated use of multiple pathways. The citric acid cycle, transport of oxaloacetate from the mitochondria, and pentose phosphate pathway provide the carbon atoms and reducing power, whereas glycolysis and oxidative phosphorylation provide the ATP to meet the needs for fatty acid synthesis (Figure 22.30).
Fatty acid metabolism is altered in tumor cells
 We have previously seen that cancer cells alter glucose metabolism to meet the needs of rapid cell growth. Cancer cells must also increase fatty acid synthesis for use as signal molecules as well as for incorporation into membrane phospholipids. Many of the enzymes of fatty acid synthesis are overexpressed in most human cancers, and this expression is correlated with tumor malignancy. Recall that normal cells do little de novo fatty acid synthesis, relying instead on dietary intake to meet their fatty needs.
We have previously seen that cancer cells alter glucose metabolism to meet the needs of rapid cell growth. Cancer cells must also increase fatty acid synthesis for use as signal molecules as well as for incorporation into membrane phospholipids. Many of the enzymes of fatty acid synthesis are overexpressed in most human cancers, and this expression is correlated with tumor malignancy. Recall that normal cells do little de novo fatty acid synthesis, relying instead on dietary intake to meet their fatty needs.
668
The dependence of de novo fatty acid synthesis provides possible therapeutic targets to inhibit cancer cell growth. Inhibition of β-ketoacyl ACP synthase, the enzyme that catalyzes the condensation step of fatty acid synthesis, does indeed inhibit phospholipid synthesis and subsequent cell growth in some cancers, apparently by inducing apoptosis. However, another startling observation was made: mice treated with inhibitors of the β-ketoacyl ACP synthase showed remarkable weight loss because they ate less. Thus, fatty acid synthase inhibitors are exciting candidates both as antitumor and as antiobesity drugs.
Acetyl CoA carboxylase is also being investigated as a possible target for inhibiting cancer cell growth. Inhibition of the carboxylase in prostate and breast cancer cell lines induces apoptosis in the cancer cells, and yet is without effect in normal cells (problem 54). Understanding the alteration of fatty acid metabolism in cancer cells is a developing area of research that holds promise of generating new cancer therapies.