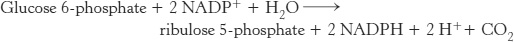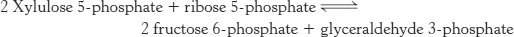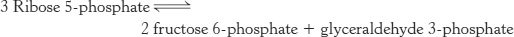20.3
The Pentose Phosphate Pathway Generates NADPH and Synthesizes Five-Carbon Sugars
TABLE 20.2 Pathways requiring NADPH
|
|
|
|
|
|
Neurotransmitter biosynthesis |
|
|
|
|
Reduction of oxidized glutathione |
Cytochrome P450 monooxygenases |
Photosynthetic organisms can use the light reactions for generation of some NADPH. Another pathway, present in all organisms, meets the NADPH needs of nonphotosynthetic organisms and of the nonphotosynthetic tissues in plants. The pentose phosphate pathway, which occurs in the cytoplasm, is a crucial source of NADPH for use in reductive biosynthesis (Table 20.2) as well as for protection against oxidative stress. This pathway consists of two phases: (1) the oxidative generation of NADPH and (2) the nonoxidative interconversion of sugars (Figure 20.18). In the oxidative phase, NADPH is generated when glucose 6-phosphate is oxidized to ribulose 5-phosphate.
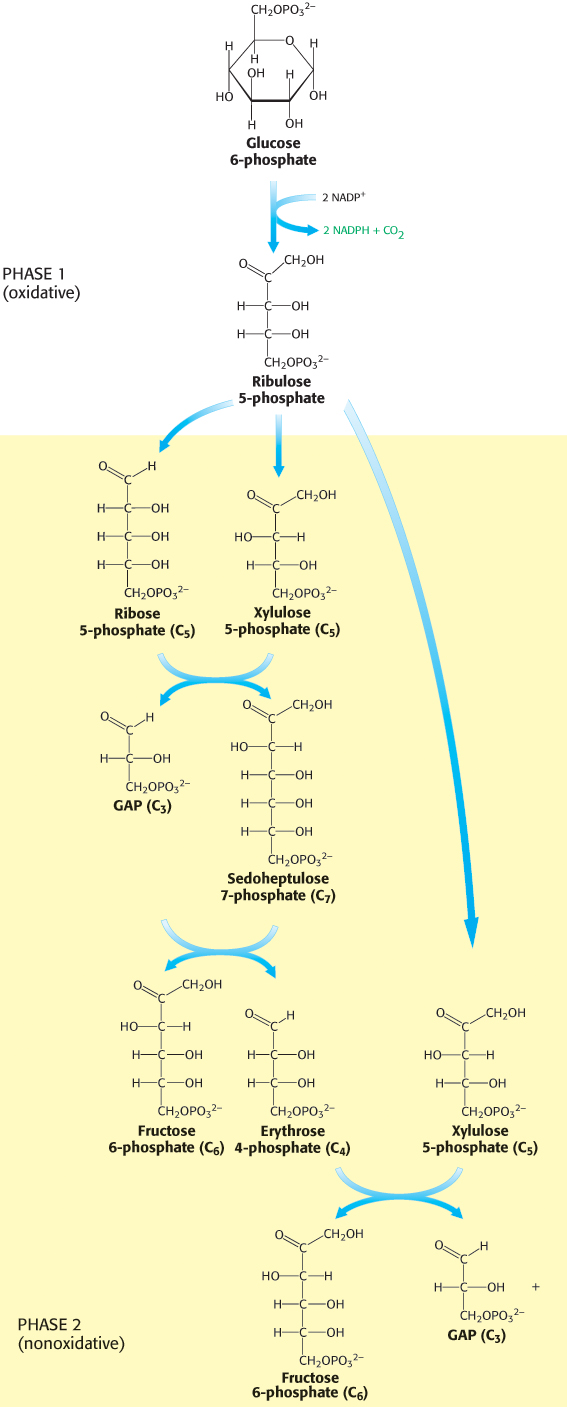
FIGURE 20.18Pentose phosphate pathway. The pathway consists of (1) an oxidative phase that generates NADPH and (2) a nonoxidative phase that interconverts phosphorylated sugars.
In the nonoxidative phase, the pathway catalyzes the interconversion of three-, four-, five-, six-, and seven-carbon sugars in a series of nonoxidative reactions. Excess five-carbon sugars may be converted into intermediates of the glycolytic pathway. All these reactions take place in the cytoplasm. These interconversions rely on the same reactions that lead to the regeneration of ribulose 1,5-bisphosphate in the Calvin cycle.
Two molecules of NADPH are generated in the conversion of glucose 6-phosphate into ribulose 5-phosphate
The oxidative phase of the pentose phosphate pathway starts with the dehydrogenation of glucose 6-phosphate at carbon 1, a reaction catalyzed by glucose 6-phosphate dehydrogenase (Figure 20.19). This enzyme is highly specific for NADP+; the KM for NAD+ is about a thousand times as great as that for NADP+. The product is 6-phosphoglucono-δ-lactone, which is an intramolecular ester between the C-1 carboxyl group and the C-5 hydroxyl group. The next step is the hydrolysis of 6-phosphoglucono-δ-lactone by a specific lactonase to give 6-phosphogluconate. This six-carbon sugar acid is then oxidatively decarboxylated by 6-phosphogluconate dehydrogenase to yield ribulose 5-phosphate. NADP+is again the electron acceptor.
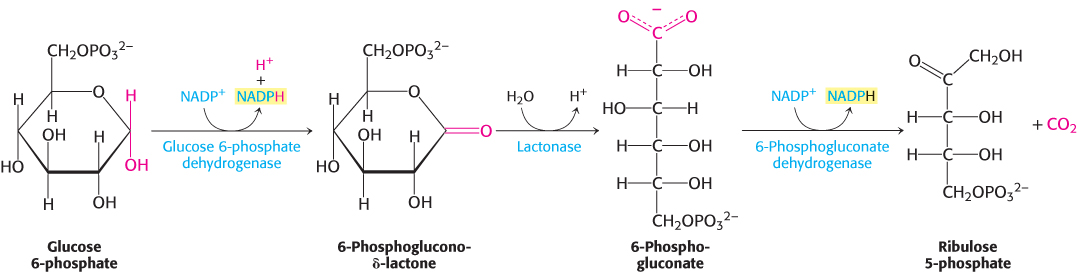
FIGURE 20.19Oxidative phase of the pentose phosphate pathway. Glucose 6-phosphate is oxidized to 6-phosphoglucono-δ-lactone to generate one molecule of NADPH. The lactone product is hydrolyzed to 6-phosphogluconate, which is oxidatively decarboxylated to ribulose 5-phosphate with the generation of a second molecule of NADPH.
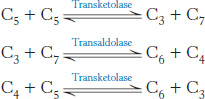
The pentose phosphate pathway and glycolysis are linked by transketolase and transaldolase
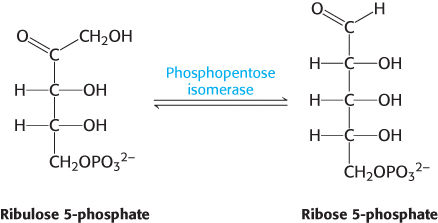
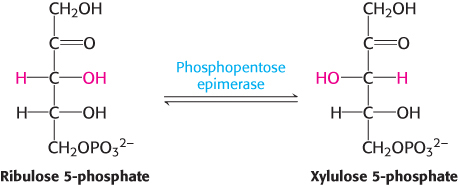
The preceding reactions yield two molecules of NADPH and one molecule of ribulose 5-phosphate for each molecule of glucose 6-phosphate oxidized. The ribulose 5-phosphate is subsequently isomerized to ribose 5-phosphate by phosphopentose isomerase.
Ribose 5-phosphate and its derivatives are components of RNA and DNA, as well as of ATP, NADH, FAD, and coenzyme A. Although ribose 5-phosphate is a precursor to many biomolecules, many cells need NADPH for reductive biosyntheses much more than they need ribose 5-phosphate for incorporation into nucleotides. For instance, adipose tissue, the liver, and mammary glands require large amounts of NADPH for fatty acid synthesis (Chapter 22). In these cases, ribose 5-phosphate is converted into the glycolytic intermediates glyceraldehyde 3-phosphate and fructose 6-phosphate by transketolase and transaldolase. These enzymes create a reversible link between the pentose phosphate pathway and glycolysis by catalyzing these three successive reactions.
The net result of these reactions is the formation of two hexoses and one triose from three pentoses:
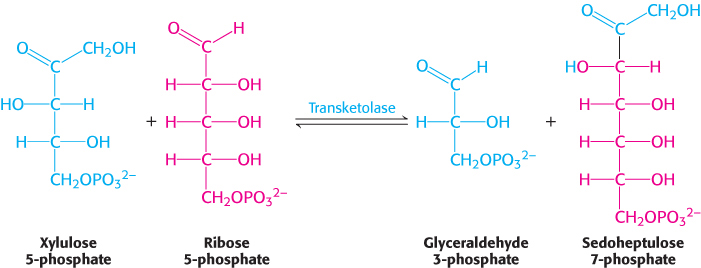
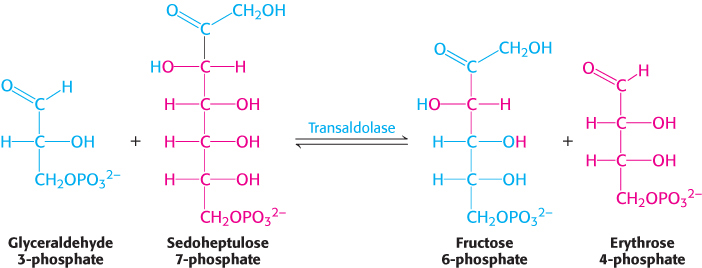
The first of the three reactions linking the pentose phosphate pathway and glycolysis is the formation of glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate from two pentoses.
The donor of the two-carbon unit in this reaction is xylulose 5-phosphate, an epimer of ribulose 5-phosphate. A ketose is a substrate of transketolase only if its hydroxyl group at C-3 has the configuration of xylulose rather than ribulose. Ribulose 5-phosphate is converted into the appropriate epimer for the transketolase reaction by phosphopentose epimerase in the reverse reaction of that which takes place in the Calvin cycle.
Glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate generated by the transketolase then react to form fructose 6-phosphate and erythrose 4-phosphate.
This synthesis of a four-carbon sugar and a six-carbon sugar is catalyzed by transaldolase.
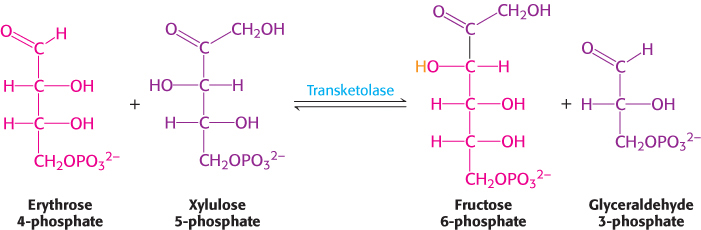
In the third reaction, transketolase catalyzes the synthesis of fructose 6-phosphate and glyceraldehyde 3-phosphate from erythrose 4-phosphate and xylulose 5-phosphate.
The sum of these reactions is
Xylulose 5-phosphate can be formed from ribose 5-phosphate by the sequential action of phosphopentose isomerase and phosphopentose epimerase, and so the net reaction starting from ribose 5-phosphate is
Thus, excess ribose 5-phosphate formed by the pentose phosphate pathway can be completely converted into glycolytic intermediates. Moreover, any ribose ingested in the diet can be processed into glycolytic intermediates by this pathway. It is evident that the carbon skeletons of sugars can be extensively rearranged to meet physiological needs (Table 20.3).
TABLE 20.3 Pentose phosphate pathway
|
|
|
|
|
|
Glucose 6-phosphate + NADP+ → 6-phosphoglucono-δ-lactone + NADPH + H+ |
Glucose 6-phosphate dehydrogenase |
6-Phosphoglucono-δ-lactone + H2O → 6-phosphogluconate + H+ |
|
6-Phosphogluconate + NADP+ → ribulose 5-phosphate + CO2 + NADPH + H+ |
6-Phosphogluconate dehydrogenase |
|
|
|
Ribulose 5-phosphate ⇋ ribose 5-phosphate |
|
Ribulose 5-phosphate ⇋ xylulose 5-phosphate |
|
Xylulose 5-phosphate + ribose 5-phosphate ⇋ Transketolase sedoheptulose 7-phosphate + glyceraldehyde 3-phosphate
|
|
Sedoheptulose 7-phosphate + glyceraldehyde 3-phosphate ⇋ Transaldolase fructose 6-phosphate + erythrose 4-phosphate
|
|
Xylulose 5-phosphate + erythrose 4-phosphate ⇋ Transketolase fructose 6-phosphate + glyceraldehyde 3-phosphate
|
|
Mechanism: Transketolase and transaldolase stabilize carbanionic intermediates by different mechanisms
The reactions catalyzed by transketolase and transaldolase are distinct, yet similar in many ways. One difference is that transketolase transfers a two-carbon unit, whereas transaldolase transfers a three-carbon unit. Each of these units is transiently attached to the enzyme in the course of the reaction and thus these enzymes are examples of double displacement reactions (Section 8.4).
Transketolase reaction. Transketolase contains a tightly bound thiamine pyrophosphate as its prosthetic group. The enzyme transfers a two-carbon glycoaldehyde from a ketose donor to an aldose acceptor. The site of the addition of the two-carbon unit is the thiazole ring of TPP. Transketolase is homologous to the E1 subunit of the pyruvate dehydrogenase complex (Section 17.1) and the reaction mechanism is similar (Figure 20.20).
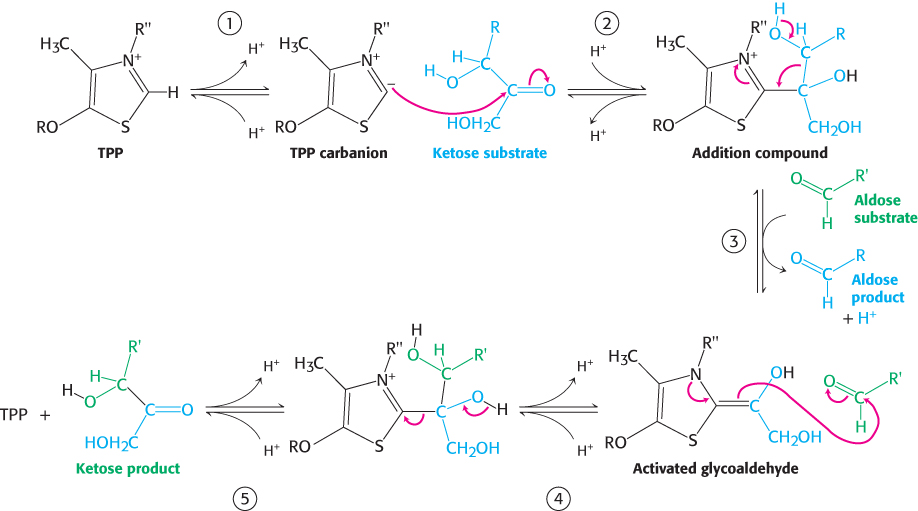
FIGURE 20.20Transketolase mechanism. (1) Thiamine pyrophosphate (TPP) ionizes to form a carbanion. (2) The carbanion of TPP attacks the ketose substrate. (3) Cleavage of a carbon–carbon bond frees the aldose product and leaves a two-carbon fragment joined to TPP. (4) This activated glycoaldehyde intermediate attacks the aldose substrate to form a new carbon–carbon bond. (5) The ketose product is released, freeing the TPP for the next reaction cycle.
The reaction proceeds as follows:
The C-2 carbon atom of bound TPP readily ionizes to give a carbanion.
The negatively charged carbon atom of this reactive intermediate attacks the carbonyl group of the ketose substrate.
The resulting addition compound releases the aldose product to yield an activated glycoaldehyde unit.
The positively charged nitrogen atom in the thiazole ring acts as an electron sink in the development of this activated intermediate. The carbonyl group of a suitable aldose acceptor then condenses with the activated glycoaldehyde unit to form a new ketose.
The ketose is then released from the enzyme.
Transaldolase reaction. Transaldolase transfers a three-carbon dihydroxyacetone unit from a ketose donor to an aldose acceptor. Transaldolase, in contrast to transketolase, does not contain a prosthetic group. Rather, a Schiff base is formed between the carbonyl group of the ketose substrate and the ε-amino group of a lysine residue at the active site of the enzyme (Figure 20.21). This kind of covalent enzyme–substrate intermediate is like that formed in fructose 1,6-bisphosphate aldolase in the glycolytic pathway (Section 16.1) and, indeed, the enzymes are homologous. (1) The reaction begins with the formation of the Schiff base. (2) The Schiff base becomes protonated and the bond between C-3 and C-4 is split. (3) Upon reprotonation, the aldose product is released leaving a three-carbon fragment bound to the enzyme. The negative charge on the Schiff-base carbanion moiety is stabilized by resonance (Figure 20.22). The positively charged nitrogen atom of the protonated Schiff base acts as an electron sink. (4) The Schiff-base adduct is stable until a suitable aldose becomes bound. (5) The dihydroxyacetone moiety then reacts with the carbonyl group of the aldose. Protonation allows the formation of a new carbon–carbon bond. (6) Subsequent deprotonation followed by (7) hydrolysis of the Schiff base releases the ketose product. The nitrogen atom of the protonated Schiff base plays the same role in transaldolase as the thiazole-ring nitrogen atom does in transketolase. In each enzyme, a group within an intermediate reacts like a carbanion in attacking a carbonyl group to form a new carbon–carbon bond. In each case, the charge on the carbanion is stabilized by resonance (Figure 20.22).
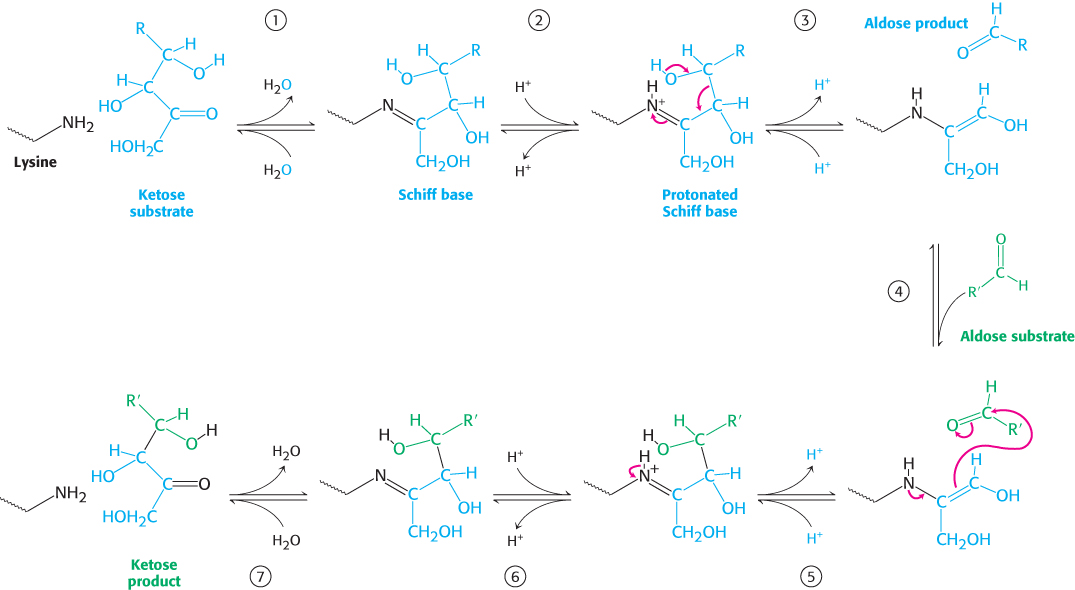
FIGURE 20.21Transaldolase mechanism. (1) The reaction begins with the formation of a Schiff base between a lysine residue in transaldolase and the ketose substrate. (2) Protonation of the Schiff base occurs. (3) Deprotonation leads to the release of the aldose product, leaving a three-carbon fragment attached to the lysine residue. (4) This intermediate adds to the aldose substrate. (5) Protonation occurs, forming a new carbon–carbon bond. (6) Subsequent deprotonation and (7) hydrolysis of the Schiff base releases the ketose product from the lysine side chain, completing the reaction cycle.
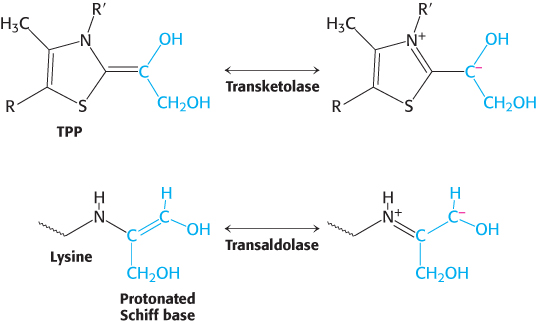
FIGURE 20.22Carbanion intermediates. For transketolase and transaldolase, a carbanion intermediate is stabilized by resonance. In transketolase, TPP stabilizes this intermediate; in transaldolase, a protonated Schiff base plays this role.