22.5The Elongation and Unsaturation of Fatty Acids Are Accomplished by Accessory Enzyme Systems
The Elongation and Unsaturation of Fatty Acids Are Accomplished by Accessory Enzyme Systems
The major product of the fatty acid synthase is palmitate. In eukaryotes, longer fatty acids are formed by elongation reactions catalyzed by enzymes on the cytoplasmic face of the endoplasmic reticulum membrane. These reactions add two-
Membrane-bound enzymes generate unsaturated fatty acids
Endoplasmic reticulum systems also introduce double bonds into long-
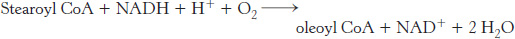
This reaction is catalyzed by a complex of three membrane-
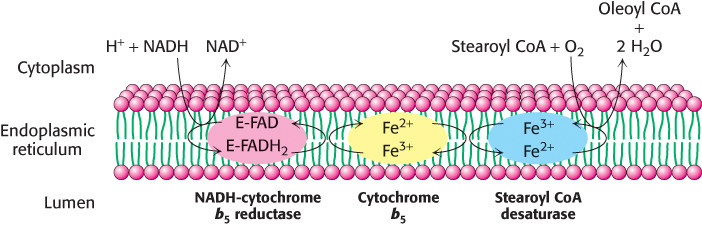
669
A variety of unsaturated fatty acids can be formed from oleate by a combination of elongation and desaturation reactions. For example, oleate can be elongated to a 20:1 cis-Δ11 fatty acid. Alternatively, a second double bond can be inserted to yield an 18:2 cis-Δ6, Δ9 fatty acid. Similarly, palmitate (16:0) can be oxidized to palmitoleate (16:1 cis-Δ9), which can then be elongated to mcis-vaccenate (18:1 cis-Δ11).
|
Precursor |
Formula |
|---|---|
|
Linolenate (ω-3) |
CH3—(CH2)2=CH— |
|
Linoleate (ω-6) |
CH3—(CH2)5=CH— |
|
Palmitoleate (ω-7) |
CH3—(CH2)6=CH— |
|
Oleate (ω-9) |
CH3—(CH2)8=CH— |
Unsaturated fatty acids in mammals are derived from either palmitoleate (16:1), oleate (18:1), linoleate (18:2), or linolenate (18:3). The number of carbon atoms from the ω end of a derived unsaturated fatty acid to the nearest double bond identifies its precursor.
Mammals lack the enzymes to introduce double bonds at carbon atoms beyond C-
Eicosanoid hormones are derived from polyunsaturated fatty acids
Arachidonate, a 20:4 fatty acid derived from linoleate, is the major precursor of several classes of signal molecules: prostaglandins, prostacyclins, thromboxanes, and leukotrienes (Figure 22.32).

A prostaglandin is a 20-
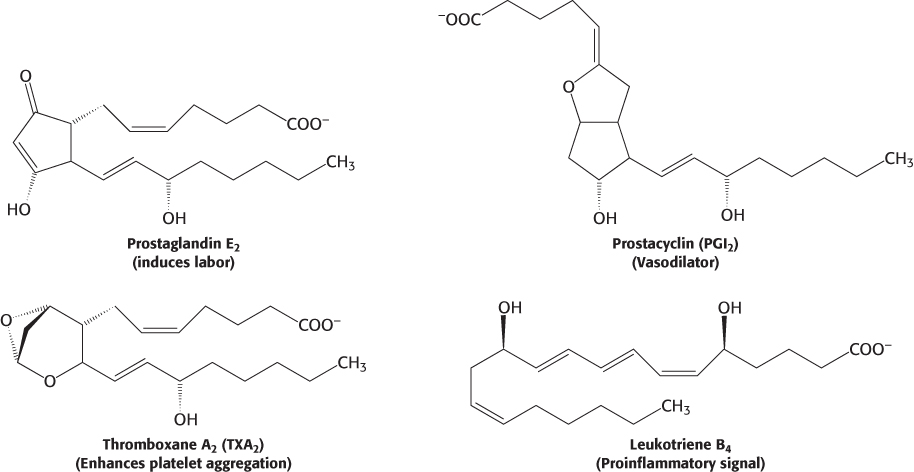
Prostaglandins and other eicosanoids are local hormones because they are short-
 Recall that aspirin blocks access to the active site of the enzyme that converts arachidonate into prostaglandin H2 (Section 12.3). Because arachidonate is the precursor of other prostaglandins, prostacyclin, and thromboxanes, blocking this step interferes with many signaling pathways. Aspirin’s ability to obstruct these pathways accounts for its wide-
Recall that aspirin blocks access to the active site of the enzyme that converts arachidonate into prostaglandin H2 (Section 12.3). Because arachidonate is the precursor of other prostaglandins, prostacyclin, and thromboxanes, blocking this step interferes with many signaling pathways. Aspirin’s ability to obstruct these pathways accounts for its wide-
670
Variations on a theme: Polyketide and nonribosomal peptide synthetases resemble fatty acid synthase
 The mammalian multifunctional fatty acid synthase is a member of a large family of complex enzymes termed megasynthases that participate in step-
The mammalian multifunctional fatty acid synthase is a member of a large family of complex enzymes termed megasynthases that participate in step-
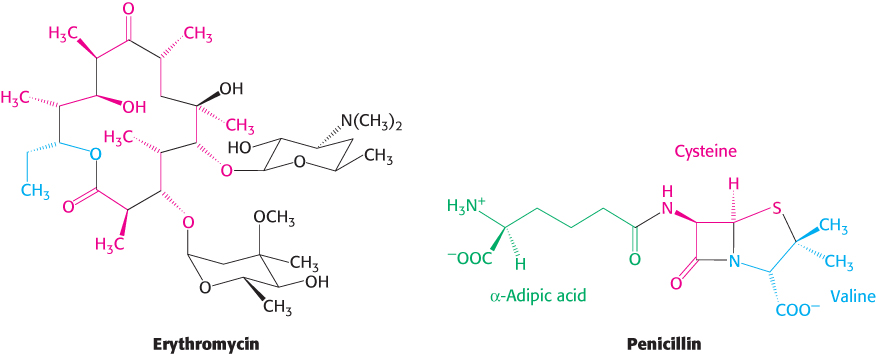
Research is underway to learn how to manipulate the pathways and the enzymes of polyketide and nonribosomal peptide synthesis in order to generate new therapeutics.