The starved–fed cycle is the physiological response to a fast
We begin with a physiological condition called the starved–fed cycle, which we all experience in the hours after an evening meal and through the night’s fast. This nightly starved–fed cycle has three stages: the well-fed state after a meal, the early fasting during the night, and the refed state after breakfast. A major goal of the many biochemical alterations in this period is to maintain glucose homeostasis—that is, a constant blood-glucose concentration. Maintaining glucose homeostasis is crucial because glucose is normally the only fuel source for the brain. As discussed earlier, the major defect in diabetes is the inability to perform this vital task. The two primary signals regulating the starved–fed cycle are insulin and glucagon.
1. The well-fed, or postprandial, state. After we consume and digest an evening meal, glucose and amino acids are transported from the intestine to the blood. The dietary lipids are packaged into chylomicrons and transported to the blood by the lymphatic system. This fed condition leads to the secretion of insulin, which signals the fed state. Insulin stimulates glycogen synthesis in both muscle and the liver, suppresses gluconeogenesis by the liver, and stimulates protein synthesis. Insulin also accelerates glycolysis in the liver, which in turn increases the synthesis of fatty acids.
The liver helps to limit the amount of glucose in the blood during times of plenty by storing it as glycogen so as to be able to release glucose in times of scarcity. How is the excess blood glucose present after a meal removed? The liver is able to trap large quantities of glucose because it possesses an isozyme of hexokinase called glucokinase, which converts glucose into glucose 6-phosphate, which cannot be transported out of the cell. Recall that glucokinase has a high KM value and is thus active only when blood-glucose concentration is high (Section 16.2). Furthermore, glucokinase is not inhibited by glucose 6-phosphate as hexokinase is. Consequently, the liver forms glucose 6-phosphate more rapidly as the blood-glucose concentration rises. The increase in glucose 6-phosphate, which activates glycogen synthase, coupled with insulin action leads to a buildup of glycogen stores. The hormonal effects on glycogen synthesis and storage are reinforced by a direct action of glucose itself. Phosphorylase a is a glucose sensor in addition to being the enzyme that cleaves glycogen. When the glucose level is high, the binding of glucose to phosphorylase a renders the enzyme susceptible to the action of a phosphatase that converts it into phosphorylase b, which does not readily degrade glycogen (Figure 21.24). Thus, glucose allosterically shifts the glycogen system from a degradative to a synthetic mode.
The high insulin level in the fed state also promotes the entry of glucose into muscle and adipose tissue. Insulin stimulates the synthesis of glycogen by muscle as well as by the liver. The entry of glucose into adipose tissue provides glycerol 3-phosphate for the synthesis of triacylglycerols. The action of insulin also extends to amino acid and protein metabolism. Insulin promotes the uptake of branched-chain amino acids (valine, leucine, and isoleucine) by muscle. Indeed, insulin has a general stimulating effect on protein synthesis, which favors a building up of muscle protein. In addition, it inhibits the intracellular degradation of proteins.
2. The early fasting, or postabsorptive, state. The blood-glucose concentration begins to drop several hours after a meal, leading to a decrease in insulin secretion and a rise in glucagon secretion by the α cells of the pancreas. The regulation of glucagon secretion is poorly understood, but when glucose is abundant, β cells inhibit glucagon secretion. When glucose concentration falls, the inhibition is relieved and glucagon is secreted by the α cells of the pancreas. Just as insulin signals the fed state, glucagon signals the starved state, serving to mobilize glycogen stores when there is no dietary intake of glucose. The main target organ of glucagon is the liver. Glucagon stimulates glycogen breakdown and inhibits glycogen synthesis by triggering the cyclic AMP cascade leading to the phosphorylation and activation of phosphorylase and the inhibition of glycogen synthase (Section 21.5). Glucagon also inhibits fatty acid synthesis by diminishing the production of pyruvate and by maintaining the inactive phosphorylated state of acetyl CoA carboxylase. In addition, glucagon stimulates gluconeogenesis in the liver and blocks glycolysis by lowering the level of F-2, 6-BP (Figure 27.9).
All known actions of glucagon are mediated by protein kinases that are activated by cyclic AMP. The activation of the cyclic AMP cascade results in a higher level of phosphorylase a activity and a lower level of glycogen synthase a activity. Glucagon’s effect on this cascade is reinforced by the low concentration of glucose in the blood. The diminished binding of glucose to phosphorylase a makes the enzyme less susceptible to the hydrolytic action of the phosphatase. Instead, the phosphatase remains bound to phosphorylase a, and so the synthase stays in the inactive phosphorylated form. Consequently, there is a rapid mobilization of glycogen.
The large amount of glucose formed by the hydrolysis of glucose 6-phosphate derived from glycogen is then released from the liver into the blood. The entry of glucose into muscle and adipose tissue decreases in response to a low insulin level. The diminished utilization of glucose by muscle and adipose tissue also contributes to the maintenance of the blood-glucose concentration. The net result of these actions of glucagon is to markedly increase the release of glucose by the liver. Both muscle and the liver use fatty acids as fuel when the blood-glucose concentration drops, saving the glucose for use by the brain and red blood cells. Thus, the blood-glucose concentration is kept at or above 4.4 mM by three major factors: (1) the mobilization of glycogen and the release of glucose by the liver, (2) the release of fatty acids by adipose tissue, and (3) the shift in the fuel used from glucose to fatty acids by muscle and the liver.
What is the result of the depletion of the liver’s glycogen stores? Gluconeogenesis from lactate and alanine continues, but this process merely replaces glucose that had already been converted into lactate and alanine by tissues such as muscle and red blood cells. Moreover, the brain oxidizes glucose completely to CO2 and H2O. Thus, for the net synthesis of glucose to take place, another source of carbon is required. Glycerol released from adipose tissue on lipolysis provides some of the carbon atoms, with the remaining carbon atoms coming from the hydrolysis of muscle proteins.
3. The refed state. What are the biochemical responses to a hearty breakfast? Fat is processed exactly as it is processed in the normal fed state. However, that is not the case for glucose. The liver does not initially absorb glucose from the blood, but, instead, leaves it for the other tissues. Moreover, the liver remains in a gluconeogenic mode. Now, however, the newly synthesized glucose is used to replenish the liver’s glycogen stores. As the blood-glucose concentration continues to rise, the liver completes the replenishment of its glycogen stores and begins to process the remaining excess glucose for fatty acid synthesis.
Metabolic adaptations in prolonged starvation minimize protein degradation
Earlier, we considered the metabolic results of overnutrition, a condition becoming all too common in prosperous nations. Let us now examine the opposite extreme. What are the adaptations if fasting is prolonged to the point of starvation, a circumstance affecting nearly a billion people worldwide? A typical well-nourished 70-kg man has fuel reserves totaling about 670,000 kJ (161,000 kcal; Table 27.4). The energy need for a 24-hour period ranges from about 6700 kJ (1600 kcal) to 25,000 kJ (6000 kcal), depending on the extent of activity. Thus, stored fuels suffice to meet caloric needs in starvation for 1 to 3 months. However, the carbohydrate reserves are exhausted in only a day.
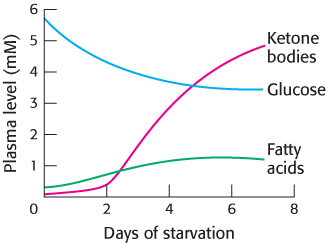
FIGURE 27.13Fuel choice during starvation. The plasma levels of fatty acids and ketone bodies increase in starvation, whereas that of glucose decreases.
TABLE 27.4 Fuel reserves in a typical 70-kg man
|
|
Available energy in kilojoules (kcal) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Data from G. F. Cahill, Jr., Clin. Endocrinol. Metab. 5:398, 1976. |
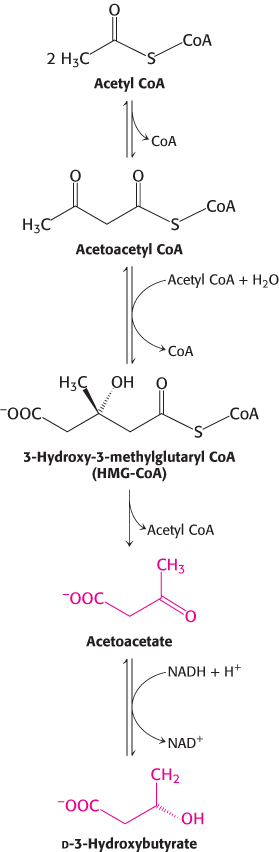
FIGURE 27.14Synthesis of ketone bodies by the liver.
Even under starvation conditions, the blood-glucose concentration must be maintained above 2.2 mM. The first priority of metabolism in starvation is to provide sufficient glucose to the brain and other tissues (such as red blood cells) that are absolutely dependent on this fuel. However, precursors of glucose are not abundant. Most energy is stored in the fatty acyl moieties of triacylglycerols. Moreover, recall that fatty acids cannot be converted into glucose, because acetyl CoA resulting from fatty acid breakdown cannot be transformed into pyruvate (Section 22.3). The glycerol moiety of triacylglycerol can be converted into glucose, but only a limited amount is available. The only other potential source of glucose is the carbon skeletons of amino acids derived from the breakdown of proteins. However, proteins are not stored, and so any breakdown will necessitate a loss of function. Thus, the second priority of metabolism in starvation is to preserve protein, which is accomplished by shifting the fuel being used from glucose to fatty acids and ketone bodies (Figure 27.13).
The metabolic changes on the first day of starvation are like those after an overnight fast. The low blood-sugar level leads to decreased secretion of insulin and increased secretion of glucagon. The dominant metabolic processes are the mobilization of triacylglycerols in adipose tissue and gluconeogenesis by the liver. The liver obtains energy for its own needs by oxidizing fatty acids released from adipose tissue. The concentrations of acetyl CoA and citrate consequently increase, which switches off glycolysis. The uptake of glucose by muscle is markedly diminished because of the low insulin level, whereas fatty acids enter freely. Consequently, muscle uses no glucose and relies exclusively on fatty acids for fuel. The β oxidation of fatty acids by muscle halts the conversion of pyruvate into acetyl CoA, because acetyl CoA stimulates the phosphorylation of the pyruvate dehydrogenase complex, which renders it inactive (Section 17.3). Hence, any available pyruvate, lactate, and alanine are exported to the liver for conversion into glucose. Glycerol derived from the cleavage of triacylglycerols is another raw material for the synthesis of glucose by the liver.
Proteolysis also provides carbon skeletons for gluconeogenesis. During starvation, degraded proteins are not replenished and serve as carbon sources for glucose synthesis. Initial sources of protein are those that turn over rapidly, such as proteins of the intestinal epithelium and the secretions of the pancreas. Proteolysis of muscle protein provides some of the three-carbon precursors of glucose. However, survival for most animals depends on being able to move rapidly, which requires a large muscle mass, and so muscle loss must be minimized.
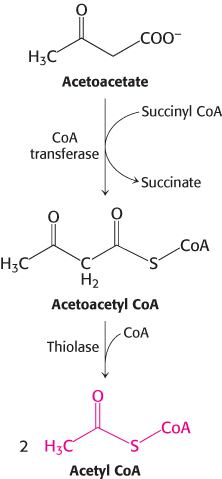
FIGURE 27.15Entry of ketone bodies into the citric acid cycle.
How is the loss of muscle curtailed? After about 3 days of starvation, the liver forms large amounts of the ketone bodies, acetoacetate and d-3-hydroxybutyrate (Figure 27.14). Their synthesis from acetyl CoA increases markedly because the citric acid cycle is unable to oxidize all the acetyl units generated by the degradation of fatty acids. Gluconeogenesis depletes the supply of oxaloacetate, which is essential for the entry of acetyl CoA into the citric acid cycle. Consequently, the liver produces large quantities of ketone bodies, which are released into the blood. At this time, the brain begins to consume significant amounts of acetoacetate in place of glucose. After 3 days of starvation, about a quarter of the energy needs of the brain are met by ketone bodies (Table 27.5). The heart also uses ketone bodies as fuel.
TABLE 27.5 Fuel metabolism in starvation
|
|
Amount formed or consumed in 24 hours (grams) |
Fuel exchanges and consumption |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Muscle-protein degradation |
|
|
|
|
|
|
|
|
|
|
|
|
After several weeks of starvation, ketone bodies become the major fuel of the brain. Acetoacetate is activated by the transfer of CoA from succinyl CoA to give acetoacetyl CoA (Figure 27.15). Cleavage by thiolase then yields two molecules of acetyl CoA, which enter the citric acid cycle. In essence, ketone bodies are equivalents of fatty acids that are an accessible fuel source for the brain. Only 40 g of glucose is then needed per day for the brain, compared with about 120 g in the first day of starvation. The effective conversion of fatty acids into ketone bodies by the liver and their use by the brain markedly diminishes the need for glucose. Hence, less muscle is degraded than in the first days of starvation. The breakdown of 20 g of muscle daily compared with 75 g early in starvation is most important for survival. A person’s survival time is mainly determined by the size of the triacylglycerol depot.
What happens after depletion of the triacylglycerol stores? The only source of fuel that remains is protein. Protein degradation accelerates, and death inevitably results from a loss of heart, liver, or kidney function.


