22.3Unsaturated and Odd-Chain Fatty Acids Require Additional Steps for Degradation
Unsaturated and Odd-Chain Fatty Acids Require Additional Steps for Degradation
The β-oxidation pathway accomplishes the complete degradation of saturated fatty acids having an even number of carbon atoms. Most fatty acids have such structures because of their mode of synthesis (to be addressed later in this chapter). However, not all fatty acids are so simple. The oxidation of fatty acids containing double bonds requires additional steps, as does the oxidation of fatty acids containing an odd number of carbon atoms.
An isomerase and a reductase are required for the oxidation of unsaturated fatty acids
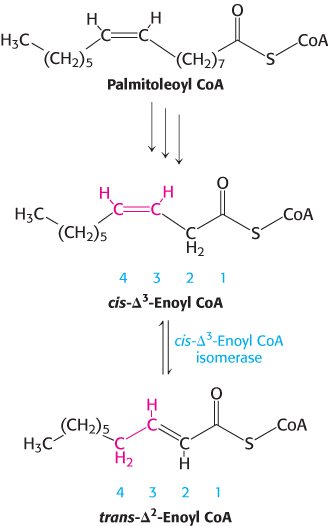
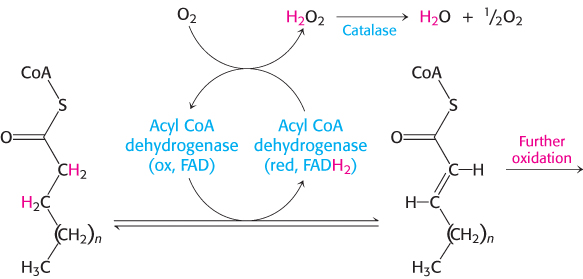
The oxidation of unsaturated fatty acids presents some difficulties, yet many such fatty acids are available in the diet. Most of the reactions are the same as those for saturated fatty acids. In fact, only two additional enzymes—
Consider the oxidation of palmitoleate (Figure 22.11). This C16 unsaturated fatty acid, which has one double bond between C-
653
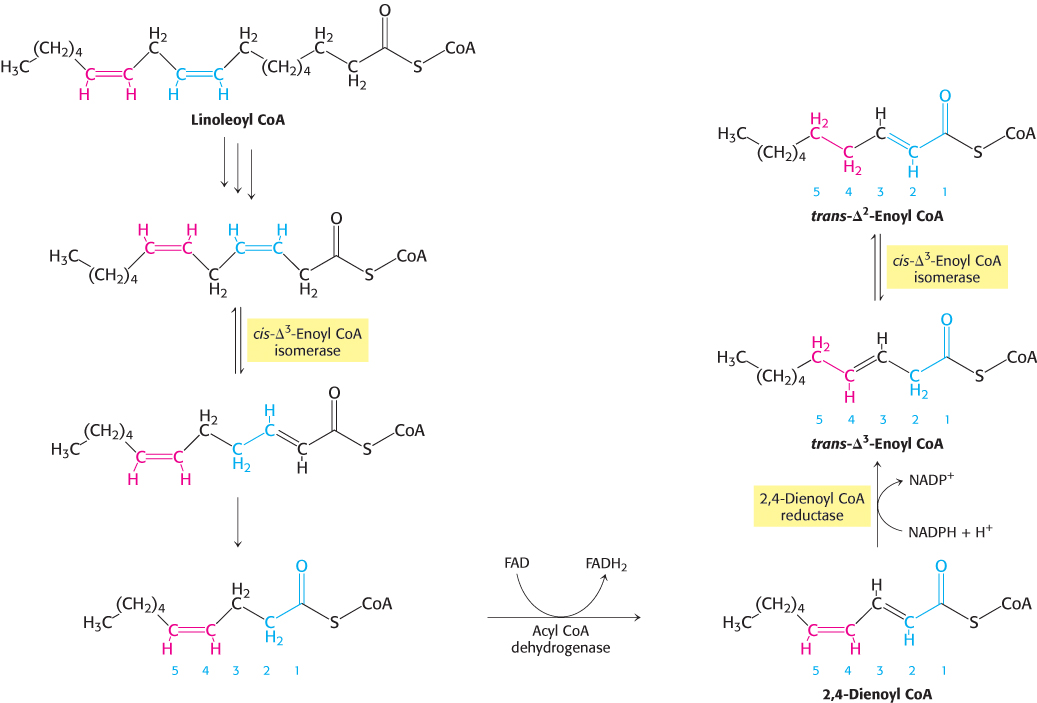
Human beings require polyunsaturated fatty acids, which have multiple double bonds, as important precursors for signal molecules, but excess polyunsaturated fatty acids are degraded by β oxidation. However, another problem arises with the oxidation of polyunsaturated fatty acids. Consider linoleate, a C18 polyunsaturated fatty acid with cis-Δ9 and cis-Δ12 double bonds (Figure 22.12). The cis-Δ3 double bond (between carbons 3 and 4) formed after three rounds of β-oxidation is converted into a trans-Δ2 double bond (between carbons 2 and 3) by the aforementioned isomerase. The acyl CoA produced by another round of β-oxidation contains a cis-Δ4 (between carbons 4 and 5) double bond. Dehydrogenation of this species by acyl CoA dehydrogenase yields a 2,4-
654
Odd-chain fatty acids yield propionyl CoA in the final thiolysis step
Fatty acids having an odd number of carbon atoms are minor species. They are oxidized in the same way as fatty acids having an even number, except that propionyl CoA and acetyl CoA, rather than two molecules of acetyl CoA, are produced in the final round of degradation. The activated three-
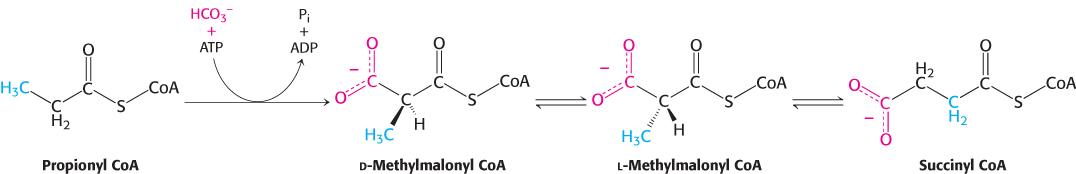
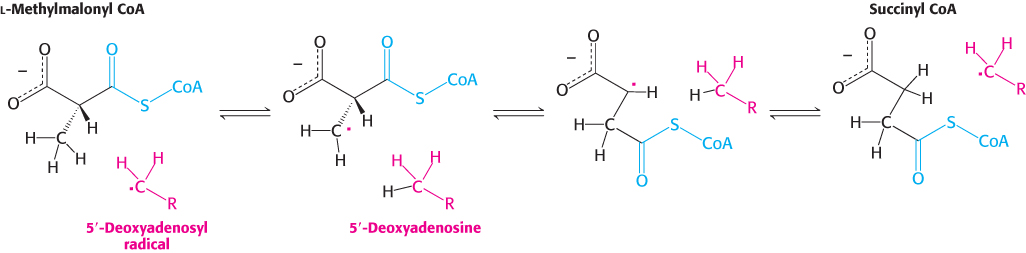
The pathway from propionyl CoA to succinyl CoA is especially interesting because it entails a rearrangement that requires vitamin B12 (also known as cobalamin). Propionyl CoA is carboxylated at the expense of the hydrolysis of a molecule of ATP to yield the d isomer of methylmalonyl CoA (Figure 22.13). This carboxylation reaction is catalyzed by propionyl CoA carboxylase, a biotin enzyme that has a catalytic mechanism like that of the homologous enzyme pyruvate carboxylase (Section 16.3). The d isomer of methylmalonyl CoA is racemized to the l isomer, the substrate for a mutase that converts it into succinyl CoA by an intramolecular rearrangement. The —CO—
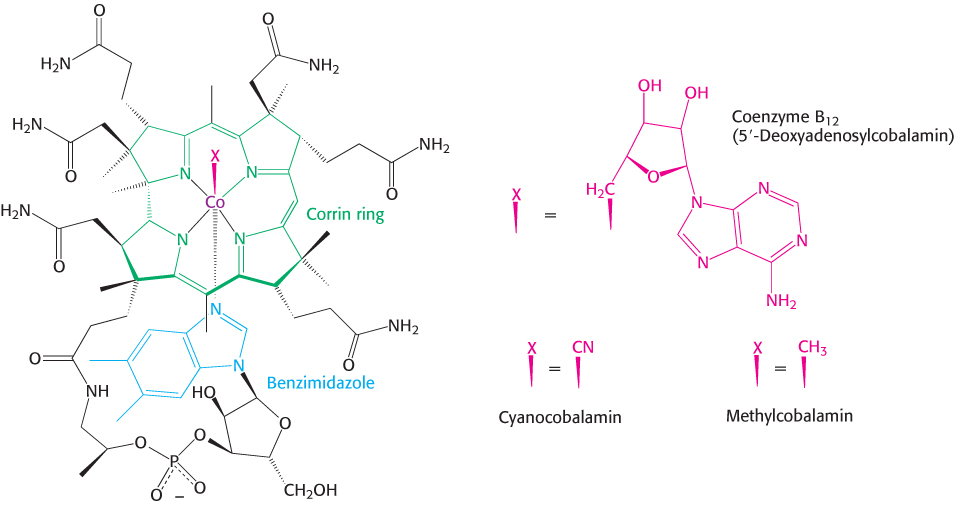
Vitamin B12 contains a corrin ring and a cobalt atom
Cobalamin enzymes, which are present in most organisms, catalyze three types of reactions: (1) intramolecular rearrangements; (2) methylations, as in the synthesis of methionine; and (3) the reduction of ribonucleotides to deoxyribonucleotides (Section 25.3). In mammals, only two reactions are known to require coenzyme B12. The conversion of l-methylmalonyl CoA into succinyl CoA is one, and the formation of methionine by the methylation of homocysteine is the other (Section 24.2). The latter reaction is especially important because methionine is required for the generation of coenzymes that participate in the synthesis of purines and thymine, which are needed for nucleic acid synthesis.
The core of cobalamin consists of a corrin ring with a central cobalt atom (Figure 22.14). The corrin ring, like a porphyrin, has four pyrrole units. Two of them are directly bonded to each other, whereas the others are joined by methine bridges, as in porphyrins. The corrin ring is more reduced than that of porphyrins and the substituents are different. A cobalt atom is bonded to the four pyrrole nitrogens. The fifth substituent linked to the cobalt atom is a derivative of dimethylbenzimidazole that contains ribose 3-
655
Mechanism: Methylmalonyl CoA mutase catalyzes a rearrangement to form succinyl CoA
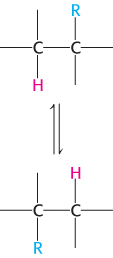
The rearrangement reactions catalyzed by coenzyme B12 are exchanges of two groups attached to adjacent carbon atoms of the substrate (Figure 22.15). A hydrogen atom migrates from one carbon atom to the next, and an R group (such as the —CO—
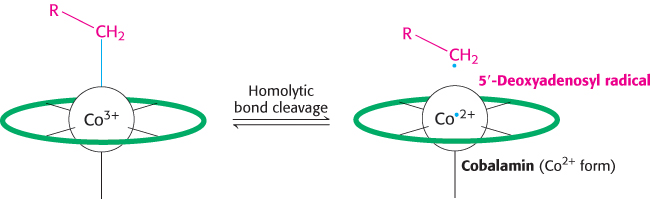
What is the role of this very unusual —CH2· radical? This highly reactive species abstracts a hydrogen atom from the substrate to form 5′-deoxyadenosine and a substrate radical (Figure 22.17). This substrate radical spontaneously rearranges: the carbonyl CoA group migrates to the position formerly occupied by H on the neighboring carbon atom to produce a different radical. This product radical abstracts a hydrogen atom from the methyl group of 5′-deoxyadenosine to complete the rearrangement and return the deoxyadenosyl unit to the radical form. The role of coenzyme B12 in such intramolecular migrations is to serve as a source of free radicals for the abstraction of hydrogen atoms.
656
An essential property of coenzyme B12 is the weakness of its cobalt–
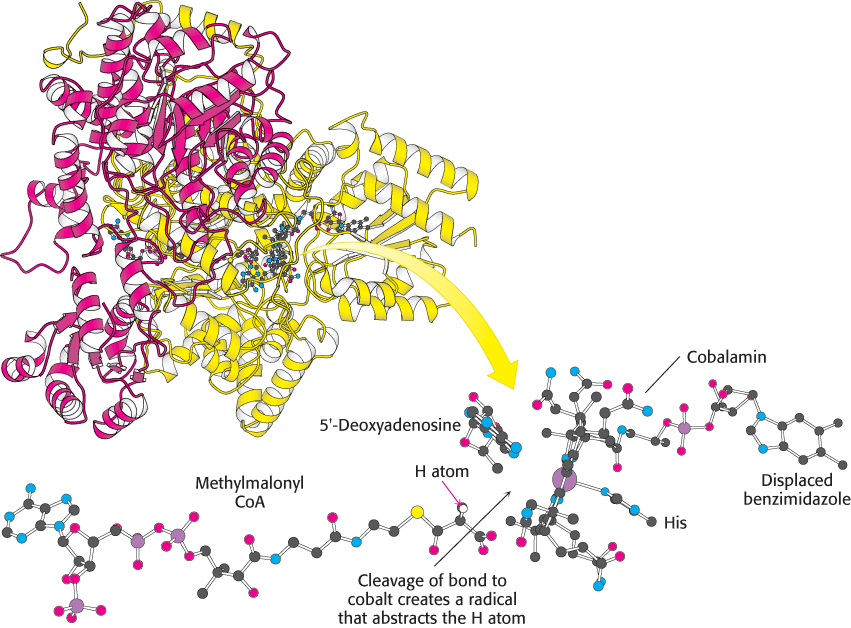
 FIGURE 22.18 Active site of methylmalonyl CoA mutase. Notice that a histidine residue from the enzyme binds to cobalt in place of benzimidazole. This arrangement of substrate and coenzyme in the active site facilitates the cleavage of the cobalt–
FIGURE 22.18 Active site of methylmalonyl CoA mutase. Notice that a histidine residue from the enzyme binds to cobalt in place of benzimidazole. This arrangement of substrate and coenzyme in the active site facilitates the cleavage of the cobalt–Fatty acids are also oxidized in peroxisomes
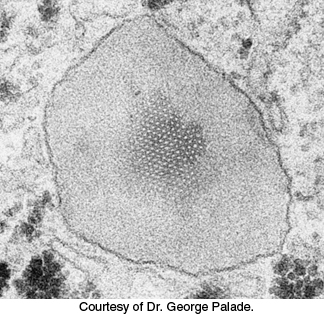
Although most fatty acid oxidation takes place in mitochondria, oxidation of long chain and branched fatty acids takes place in cellular organelles called peroxisomes (Figure 22.19) (problems 17 and 44). These organelles are small membrane-
657
 Peroxisomes do not function in patients with Zellweger syndrome. Liver, kidney, and muscle abnormalities usually lead to death by age 6. The syndrome is caused by a defect in the import of enzymes into the peroxisomes. Here we see a pathological condition resulting from an inappropriate cellular distribution of enzymes.
Peroxisomes do not function in patients with Zellweger syndrome. Liver, kidney, and muscle abnormalities usually lead to death by age 6. The syndrome is caused by a defect in the import of enzymes into the peroxisomes. Here we see a pathological condition resulting from an inappropriate cellular distribution of enzymes.
Ketone bodies are formed from acetyl CoA when fat breakdown predominates
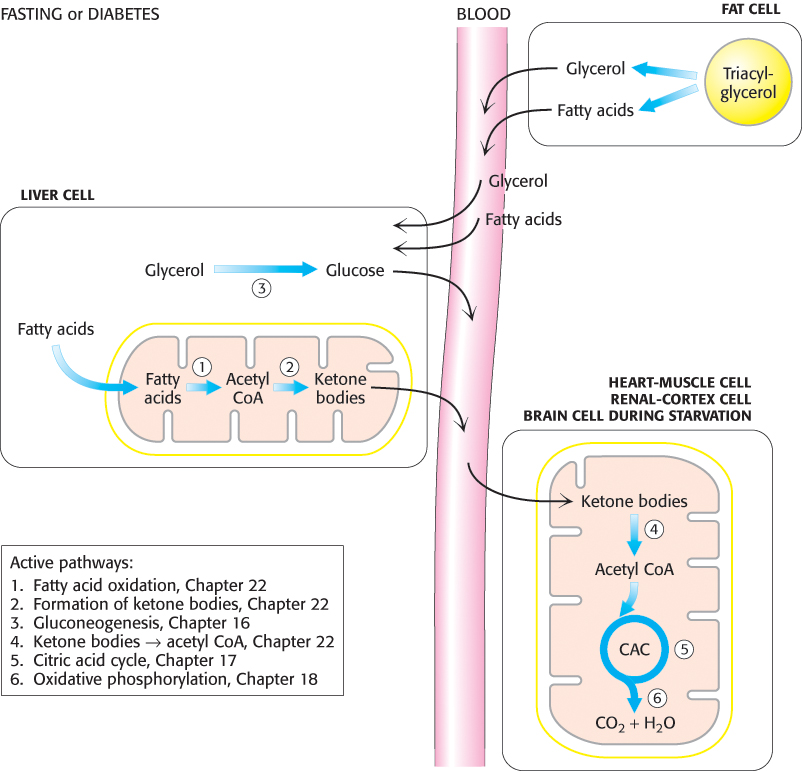
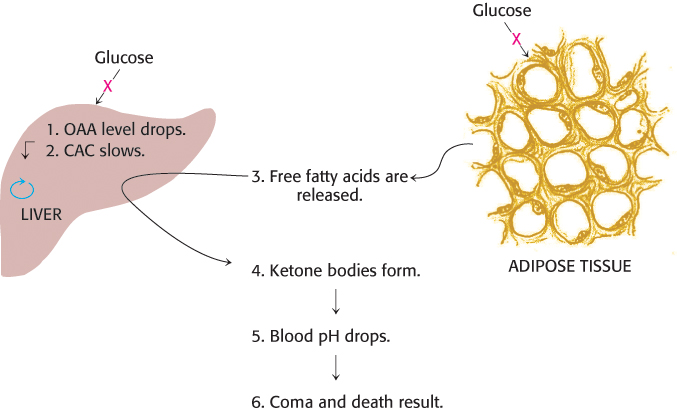
The acetyl CoA formed in fatty acid oxidation enters the citric acid cycle only if fat and carbohydrate degradation are appropriately balanced. Acetyl CoA must combine with oxaloacetate to gain entry to the citric acid cycle. The availability of oxaloacetate, however, depends on an adequate supply of carbohydrate. Recall that oxaloacetate is normally formed from pyruvate, the product of glucose degradation in glycolysis, by pyruvate carboxylase (Section 16.3). If carbohydrate is unavailable or improperly utilized, the concentration of oxaloacetate is lowered and acetyl CoA cannot enter the citric acid cycle. This dependency is the molecular basis of the adage that fats burn in the flame of carbohydrates.
In fasting or diabetes, oxaloacetate is consumed to form glucose by the gluconeogenic pathway (Section 16.3) and hence is unavailable for condensation with acetyl CoA. Under these conditions, acetyl CoA is diverted to the formation of acetoacetate and d-3-
Acetoacetate is formed from acetyl CoA in three steps (Figure 22.21). Two molecules of acetyl CoA condense to form acetoacetyl CoA. This reaction, which is catalyzed by thiolase, is the reverse of the thiolysis step in the oxidation of fatty acids. Acetoacetyl CoA then reacts with acetyl CoA and water to give 3-
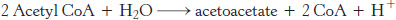
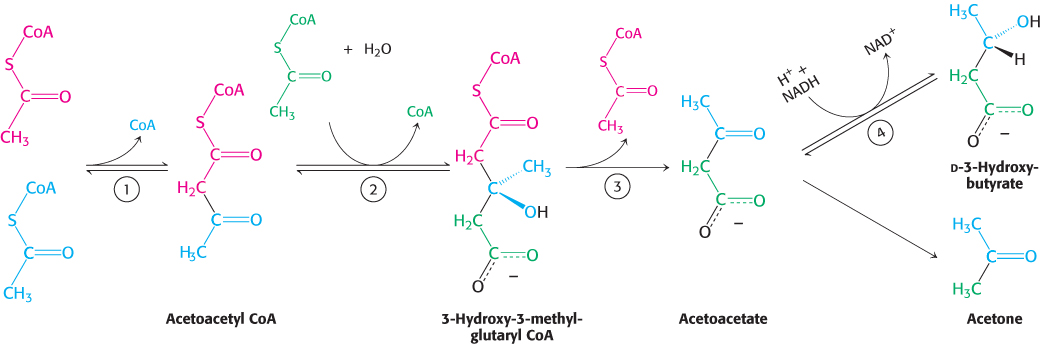
658
d-3-
Because it is a β-ketoacid, acetoacetate also undergoes a slow, spontaneous decarboxylation to acetone. The odor of acetone may be detected in the breath of a person who has a high level of acetoacetate in the blood. Under starvation conditions, the acetone may be captured to synthesize glucose.
Ketone bodies are a major fuel in some tissues
The major site of the production of acetoacetate and 3-
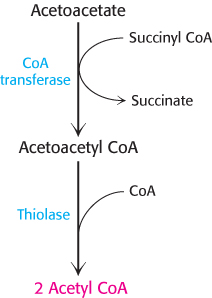
Acetoacetate is converted into acetyl CoA in two steps. First, acetoacetate is activated by the transfer of CoA from succinyl CoA in a reaction catalyzed by a specific CoA transferase. Second, acetoacetyl CoA is cleaved by thiolase to yield two molecules of acetyl CoA, which can then enter the citric acid cycle (Figure 22.23). The liver has acetoacetate available to supply to other organs because it lacks this particular CoA transferase. 3-
659
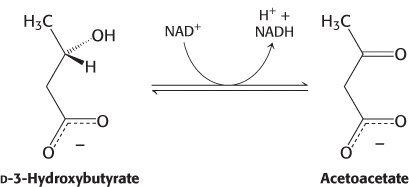
Ketone bodies can be regarded as a water-
 High blood levels of ketone bodies, the result of certain pathological conditions, can be life threatening. The most common of these conditions is diabetic ketosis in patients with insulin-
High blood levels of ketone bodies, the result of certain pathological conditions, can be life threatening. The most common of these conditions is diabetic ketosis in patients with insulin-
660
 Interestingly, diets that promote ketone-
Interestingly, diets that promote ketone-
Animals cannot convert fatty acids into glucose
A typical human being has far greater fat stores than glycogen stores. However, glycogen is necessary to fuel very active muscle, as well as the brain, which normally uses only glucose as a fuel. When glycogen stores are low, why can’t the body make use of fat stores and convert fatty acids into glucose? Because animals are unable to effect the net synthesis of glucose from fatty acids. Specifically, acetyl CoA cannot be converted into pyruvate or oxaloacetate in animals. Recall that the reaction that generates acetyl CoA from pyruvate is irreversible (Section 17.1). The two carbon atoms of the acetyl group of acetyl CoA enter the citric acid cycle, but two carbon atoms leave the cycle in the decarboxylations catalyzed by isocitrate dehydrogenase and α-ketoglutarate dehydrogenase. Consequently, oxaloacetate is regenerated, but it is not formed de novo when the acetyl unit of acetyl CoA is oxidized by the citric acid cycle. In essence, two carbon atoms enter the cycle as an acetyl group, but two carbons leave the cycle as CO2 before oxaloacetate is generated. As a result, no net synthesis of oxaloacetate is possible. In contrast, plants have two additional enzymes enabling them to convert the carbon atoms of acetyl CoA into oxaloacetate (Section 17.5).
661
Some fatty acids may contribute to the development of pathological conditions
 As we will see shortly (Section 22.5), certain polyunsaturated fatty acids are essential for life, serving as precursors to various signal molecules. Vegetable oils, used commonly in food preparation, are rich in polyunsaturated fatty acids. However, polyunsaturated fatty acids are unstable and are readily oxidized. This tendency to become rancid reduces their shelf life and renders them undesirable for cooking. To circumvent this problem, polyunsaturated fatty acids are hydrogenated, converting them to saturated and trans unsaturated fatty acids (popularly known as “trans fat”), a variety of fat that is rare in nature. Epidemiological evidence suggests that consumption of large amounts of saturated fatty acids and trans fat promotes obesity, type 2 diabetes, and atherosclerosis. The mechanism by which these fats exert these effects is under active investigation. Some evidence suggests that they promote an inflammatory response and may mute the action of insulin and other hormones (Section 27.3).
As we will see shortly (Section 22.5), certain polyunsaturated fatty acids are essential for life, serving as precursors to various signal molecules. Vegetable oils, used commonly in food preparation, are rich in polyunsaturated fatty acids. However, polyunsaturated fatty acids are unstable and are readily oxidized. This tendency to become rancid reduces their shelf life and renders them undesirable for cooking. To circumvent this problem, polyunsaturated fatty acids are hydrogenated, converting them to saturated and trans unsaturated fatty acids (popularly known as “trans fat”), a variety of fat that is rare in nature. Epidemiological evidence suggests that consumption of large amounts of saturated fatty acids and trans fat promotes obesity, type 2 diabetes, and atherosclerosis. The mechanism by which these fats exert these effects is under active investigation. Some evidence suggests that they promote an inflammatory response and may mute the action of insulin and other hormones (Section 27.3).