16.4
Gluconeogenesis and Glycolysis Are Reciprocally Regulated
Gluconeogenesis and glycolysis are coordinated so that, within a cell, one pathway is relatively inactive while the other is highly active. If both sets of reactions were highly active at the same time, the net result would be the hydrolysis of four nucleoside triphosphates (two ATP molecules plus two GTP molecules) per reaction cycle. Both glycolysis and gluconeogenesis are highly exergonic under cellular conditions, and so there is no thermodynamic barrier to such simultaneous activity. However, the amounts and activities of the distinctive enzymes of each pathway are controlled so that both pathways are not highly active at the same time. The rate of glycolysis is also determined by the concentration of glucose, and the rate of gluconeogenesis by the concentrations of lactate and other precursors of glucose. The basic premise of the reciprocal regulation is that, when energy is needed or glycolytic intermediates are needed for biosynthesis, glycolysis will predominate. When there is a surplus of energy and glucose precursors, gluconeogenesis will take over.
Energy charge determines whether glycolysis or gluconeogenesis will be most active
The first important regulation site is the interconversion of fructose 6-phosphate and fructose 1,6-bisphosphate (Figure 16.29). Consider first a situation in which energy is needed. In this case, the concentration of AMP is high. Under this condition, AMP stimulates phosphofructokinase but inhibits fructose 1,6-bisphosphatase. Thus, glycolysis is turned on and gluconeogenesis is inhibited. Conversely, high levels of ATP and citrate indicate that the energy charge is high and that biosynthetic intermediates are abundant. ATP and citrate inhibit phosphofructokinase, whereas citrate activates fructose 1,6-bisphosphatase. Under these conditions, glycolysis is nearly switched off and gluconeogenesis is promoted. Why does citrate take part in this regulatory scheme? As we will see in Chapter 17, citrate reports on the status of the citric acid cycle, the primary pathway for oxidizing fuels in the presence of oxygen. High levels of citrate indicate an energy-rich situation and the presence of precursors for biosynthesis.
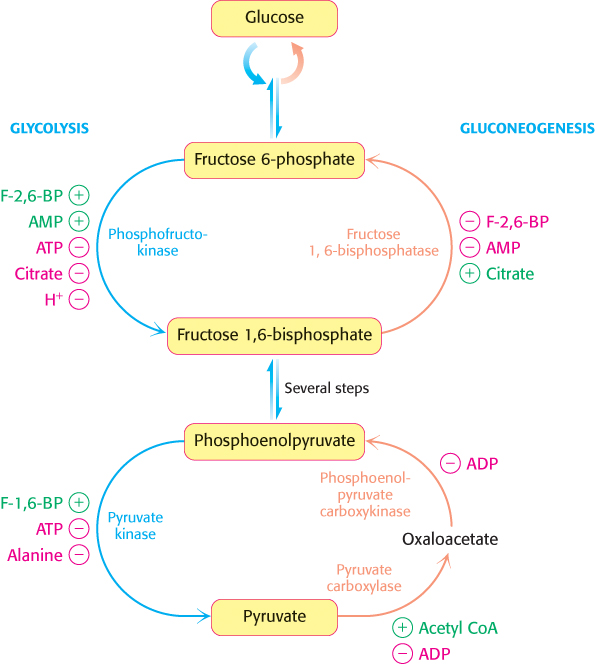
FIGURE 16.29Reciprocal regulation of gluconeogenesis and glycolysis in the liver. The level of fructose 2,6-bisphosphate is high in the fed state and low in starvation. Another important control is the inhibition of pyruvate kinase by phosphorylation during starvation.
Glycolysis and gluconeogenesis are also reciprocally regulated at the interconversion of phosphoenolpyruvate and pyruvate in the liver. The glycolytic enzyme pyruvate kinase is inhibited by allosteric effectors ATP and alanine, which signal that the energy charge is high and that building blocks are abundant. Conversely, pyruvate carboxylase, which catalyzes the first step in gluconeogenesis from pyruvate, is inhibited by ADP. Likewise, ADP inhibits phosphoenolpyruvate carboxykinase. Pyruvate carboxylase is activated by acetyl CoA, which, like citrate, indicates that the citric acid cycle is producing energy and biosynthetic intermediates (Chapter 17). Hence, gluconeogenesis is favored when the cell is rich in biosynthetic precursors and ATP.
The balance between glycolysis and gluconeogenesis in the liver is sensitive to blood-glucose concentration
In the liver, rates of glycolysis and gluconeogenesis are adjusted to maintain blood-glucose levels. The signal molecule fructose 2,6-bisphosphate strongly stimulates phosphofructokinase (PFK) and inhibits fructose 1,6-bisphosphatase. When blood glucose is low, fructose 2,6-bisphosphate loses a phosphoryl group to form fructose 6-phosphate, which is not an allosteric effector of PFK. How is the concentration of fructose 2,6-bisphosphate controlled to rise and fall with blood-glucose levels? Two enzymes regulate the concentration of this molecule: one phosphorylates fructose 6-phosphate and the other dephosphorylates fructose 2,6-bisphosphate. Fructose 2,6-bisphosphate is formed in a reaction catalyzed by phosphofructokinase 23(PFK2), a different enzyme from phosphofructokinase. Fructose 6-phosphate is formed through the hydrolysis of fructose 2,6-bisphosphate by a specific phosphatase, fructose bisphos-phatase 2 (FBPase2). The striking finding is that both PFK2 and FBPase2 are present in a single 55-kDa polypeptide chain (Figure 16.30). This bifunctional enzyme contains an N-terminal regulatory domain, followed by a kinase domain and a phosphatase domain. PFK2 resembles adenylate kinase in having a P-loop NTPase domain (Section 9.4), whereas FBPase2 resembles phosphoglycerate mutase. Recall that the mutase is essentially a phosphatase. In the bifunctional enzyme, the phosphatase activity evolved to become specific for F-2,6-BP. The bifunctional enzyme itself probably arose by the fusion of genes encoding the kinase and phosphatase domains.
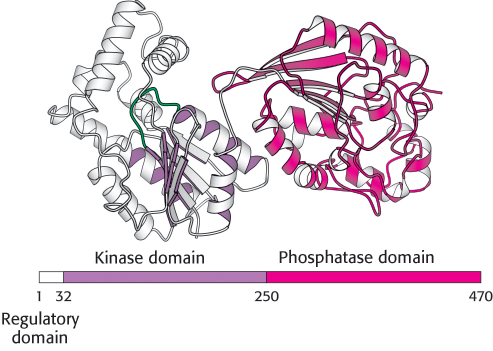
FIGURE 16.30Domain structure of the bifunctional enzyme phosphofructokinase 2. The kinase domain (purple) is fused to the phosphatase domain (red). The kinase domain is a P-loop NTP hydrolase domain, as indicated by the purple shading (Section 9.4). The bar represents the amino acid sequence of the enzyme.
[Drawn from 1BIF.pdb.]
What controls whether PFK2 or FBPase2 dominates the bifunctional enzyme’s activities in the liver? The activities of PFK2 and FBPase2 are reciprocally controlled by phosphorylation of a single serine residue. When glucose is scarce, such as during a night’s fast, a rise in the blood level of the hormone glucagon triggers a cyclic AMP signal cascade (Section 14.1), leading to the phosphorylation of this bifunctional enzyme by protein kinase A (Figure 16.31). This covalent modification activates FBPase2 and inhibits PFK2, lowering the level of F-2,6-BP. Gluconeogenesis predominates. Glucose formed by the liver under these conditions is essential for the viability of the brain. Glucagon stimulation of protein kinase A also inactivates pyruvate kinase in the liver.
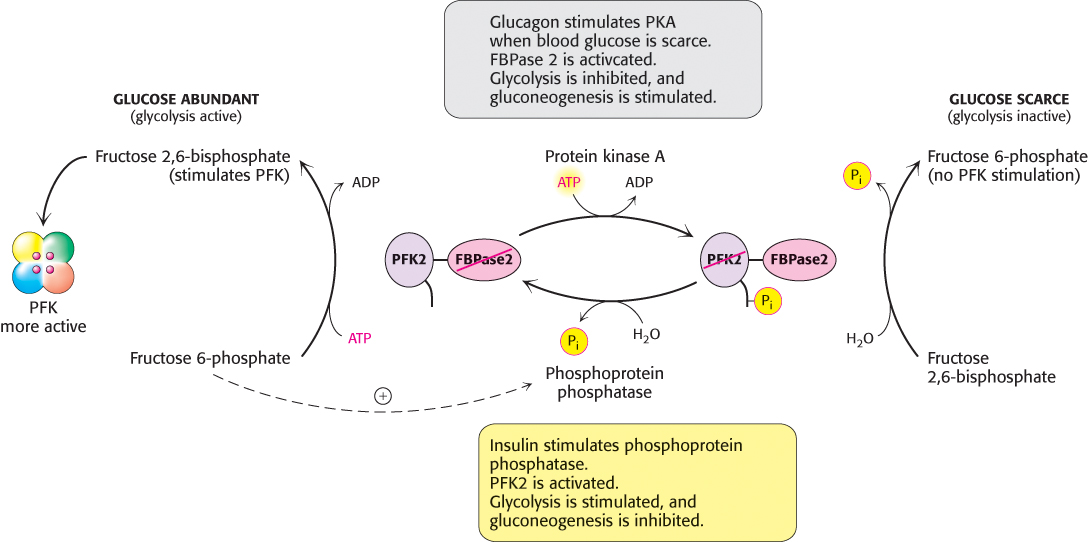
FIGURE 16.31Control of the synthesis and degradation of fructose 2,6-bisphosphate. A low blood-glucose level as signaled by glucagon leads to the phosphorylation of the bifunctional enzyme and hence to a lower level of fructose 2,6-bisphosphate, slowing glycolysis. Insulin accelerates the formation of fructose 2,6-bisphosphate by facilitating the dephosphorylation of the bifunctional enzyme.
Conversely, when blood-glucose levels are high, such as after a meal, gluconeogenesis is not needed. Insulin is secreted and initiates a signal pathway that activates a protein phosphatase, which removes the phosphoryl group from the bifunctional enzyme. This covalent modification activates PFK2 and inhibits FBPase2. The resulting rise in the level of F-2,6-BP accelerates glycolysis. In liver, a key role of glycolysis is to generate metabolites for biosynthesis. The coordinated control of glycolysis and gluconeogenesis is facilitated by the location of the kinase and phosphatase domains on the same polypeptide chain as the regulatory domain.
The hormones insulin and glucagon also regulate the amounts of essential enzymes. These hormones alter gene expression primarily by changing the rate of transcription. Insulin levels rise subsequent to eating, when there is plenty of glucose for glycolysis. To encourage glycolysis, insulin stimulates the expression of phosphofructokinase, pyruvate kinase, and the bifunctional enzyme that makes and degrades F-2,6-BP. Glucagon rises during fasting, when gluconeogenesis is needed to replace scarce glucose. To encourage gluconeogenesis, glucagon inhibits the expression of the three regulated glycolytic enzymes and stimulates instead the production of two key gluconeogenic enzymes, phosphoenolpyruvate carboxykinase and fructose 1,6-bisphosphatase. Transcriptional control in eukaryotes is much slower than allosteric control, taking hours or days instead of seconds to minutes.
Substrate cycles amplify metabolic signals and produce heat
A pair of reactions such as the phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate and its hydrolysis back to fructose 6-phosphate is called a substrate cycle. As already mentioned, both reactions are not fully active at the same time because of reciprocal allosteric controls. However, isotope-labeling studies have shown that some fructose 6-phosphate is phosphorylated to fructose 1,6-bisphosphate even during gluconeogenesis. There also is a limited degree of cycling in other pairs of opposed irreversible reactions. This cycling was regarded as an imperfection in metabolic control, and so substrate cycles have sometimes been called futile cycles. In malignant hyperthermia, there is rapid uncontrolled hydrolysis of ATP, which generates heat and can raise the body temperature to 44 °C (111°F). Muscles may become rigid and suffer substantial tissue damage.
Despite such extraordinary circumstances, substrate cycles now seem likely to be biologically important. One possibility is that substrate cycles amplify metabolic signals. Suppose that the rate of conversion of A into B is 100 and of B into A is 90, giving an initial net flux of 10. Assume that an allosteric effector increases the A → B rate by 20% to 120 and reciprocally decreases the B → A rate by 20% to 72. The new net flux is 48, and so a 20% change in the rates of the opposing reactions has led to a 380% increase in the net flux. In the example shown in Figure 16.32, this amplification is made possible by the rapid hydrolysis of ATP. The flux down the glycolytic pathway has been suggested to increase as much as 1000-fold at the initiation of intense exercise. Because the allosteric activation of enzymes alone seems unlikely to explain this increased flux, the existence of substrate cycles may partly account for the rapid rise in the rate of glycolysis.
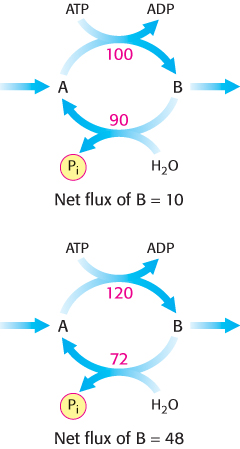
FIGURE 16.32Substrate cycle. This ATP-driven cycle operates at two different rates. A small change in the rates of the two opposing reactions results in a large change in the net flux of product B.
Lactate and alanine formed by contracting muscle are used by other organs
Lactate produced by active skeletal muscle and erythrocytes is a source of energy for other organs. Erythrocytes lack mitochondria and can never oxidize glucose completely. In contracting fast-twitch skeletal muscle fibers during vigorous exercise, the rate at which glycolysis produces pyruvate exceeds the rate at which the citric acid cycle oxidizes it. In these cells, lactate dehydrogenase reduces excess pyruvate to lactate to restore redox balance. However, lactate is a dead end in metabolism. It must be converted back into pyruvate before it can be metabolized. Both pyruvate and lactate diffuse out of these cells through carriers into the blood. In contracting skeletal muscle, the formation and release of lactate lets the muscle generate ATP in the absence of oxygen and shifts the burden of metabolizing lactate from muscle to other organs. The pyruvate and lactate in the bloodstream have two fates. In one fate, the plasma membranes of some cells, particularly cells in cardiac muscle and slow-twitch (type 1) skeletal muscle, contain carriers that make the cells highly permeable to lactate and pyruvate. These molecules diffuse from the blood into such permeable cells. Once inside these well-oxygenated cells, lactate can be reverted back to pyruvate and metabolized through the citric acid cycle and oxidative phosphorylation to generate ATP. The use of lactate in place of glucose by these cells makes more circulating glucose available to the active muscle cells. In the other fate, excess lactate enters the liver and is converted first into pyruvate and then into glucose by the gluconeogenic pathway. Contracting skeletal muscle supplies lactate to the liver, which uses it to synthesize and release glucose. Thus, the liver restores the level of glucose necessary for active muscle cells, which derive ATP from the glycolytic conversion of glucose into lactate. These reactions constitute the Cori cycle (Figure 16.33).
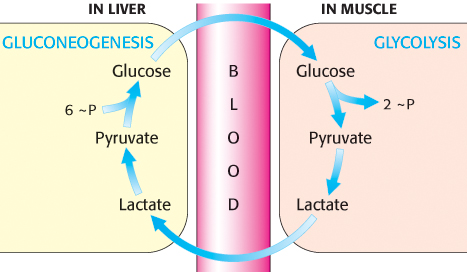
FIGURE 16.33The Cori cycle. Lactate formed by active muscle is converted into glucose by the liver. This cycle shifts part of the metabolic burden of active muscle to the liver. The symbol ∼P represents nucleoside triphosphates.
Studies have shown that alanine, like lactate, is a major precursor of glucose in the liver. The alanine is generated in muscle when the carbon skeletons of some amino acids are used as fuels. The nitrogens from these amino acids are transferred to pyruvate to form alanine; the reverse reaction takes place in the liver. This process also helps maintain nitrogen balance. The interplay between glycolysis and gluconeogenesis is summarized in Figure 16.34, which shows how these pathways help meet the energy needs of different cell types.
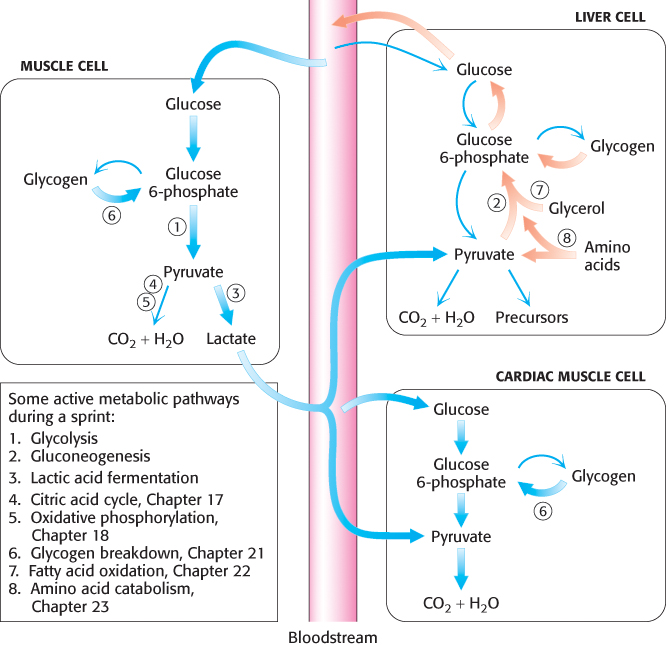
FIGURE 16.34PATHWAY INTEGRATION: Cooperation between glycolysis and gluconeogenesis during a sprint. Glycolysis and gluconeogenesis are coordinated, in a tissue-specific fashion, to ensure that the energy needs of all cells are met. Consider a sprinter. In skeletal leg muscle, glucose will be metabolized aerobically to CO2 and H2O or, more likely (thick arrows) during a sprint, anaerobically to lactate. In cardiac muscle, the lactate can be converted into pyruvate and used as a fuel, along with glucose, to power the heartbeats to keep the sprinter’s blood flowing. Gluconeogenesis, a primary function of the liver, will be taking place rapidly (thick arrows) to ensure that enough glucose is present in the blood for skeletal and cardiac muscle, as well as for other tissues. Glycogen, glycerol, and amino acids are other sources of energy that we will learn about in later chapters.
 Isozymic forms of lactate dehydrogenase in different tissues catalyze the interconversions of pyruvate and lactate (Section 10.2). Lactate dehydrogenase is a tetramer of two kinds of 35-kDa subunits encoded by similar genes: the H type predominates in the heart, and the homologous M type in skeletal muscle and the liver. These subunits associate to form five types of tetramers: H4, H3M1, H2M2, H1M3, and M4. The H4 isozyme (type 1) has higher affinity for substrates than that of the M4 isozyme (type 5) and, unlike M4, is allosterically inhibited by high levels of pyruvate. The other isozymes have intermediate properties, depending on the ratio of the two kinds of chains. The H4 isozyme oxidizes lactate to pyruvate, which is then used as a fuel by the heart through aerobic metabolism. Indeed, heart muscle never functions anaerobically. In contrast, M4 is optimized to operate in the reverse direction, to convert pyruvate into lactate to allow glycolysis to proceed under anaerobic conditions. Interestingly, endurance training increases the H4 isozyme in slow-twitch muscle fibers, enabling these fibers to use the lactate generated by other fiber types.
Isozymic forms of lactate dehydrogenase in different tissues catalyze the interconversions of pyruvate and lactate (Section 10.2). Lactate dehydrogenase is a tetramer of two kinds of 35-kDa subunits encoded by similar genes: the H type predominates in the heart, and the homologous M type in skeletal muscle and the liver. These subunits associate to form five types of tetramers: H4, H3M1, H2M2, H1M3, and M4. The H4 isozyme (type 1) has higher affinity for substrates than that of the M4 isozyme (type 5) and, unlike M4, is allosterically inhibited by high levels of pyruvate. The other isozymes have intermediate properties, depending on the ratio of the two kinds of chains. The H4 isozyme oxidizes lactate to pyruvate, which is then used as a fuel by the heart through aerobic metabolism. Indeed, heart muscle never functions anaerobically. In contrast, M4 is optimized to operate in the reverse direction, to convert pyruvate into lactate to allow glycolysis to proceed under anaerobic conditions. Interestingly, endurance training increases the H4 isozyme in slow-twitch muscle fibers, enabling these fibers to use the lactate generated by other fiber types.
Glycolysis and gluconeogenesis are evolutionarily intertwined
 The metabolism of glucose has ancient origins. Organisms living in the early biosphere depended on the anaerobic generation of energy until significant amounts of oxygen began to accumulate 2 billion years ago. Glycolytic enzymes were most likely derived independently rather than by gene duplication, because glycolytic enzymes with similar properties do not have similar amino acid sequences. Although there are four kinases and two isomerases in the pathway, sequence and structural comparisons suggest that these sets of enzymes are not related to one another by divergent evolution. The common dinucleotide-binding domain found in the dehydrogenases (Figure 16.12) and the αβ barrels are the only major recurring elements.
The metabolism of glucose has ancient origins. Organisms living in the early biosphere depended on the anaerobic generation of energy until significant amounts of oxygen began to accumulate 2 billion years ago. Glycolytic enzymes were most likely derived independently rather than by gene duplication, because glycolytic enzymes with similar properties do not have similar amino acid sequences. Although there are four kinases and two isomerases in the pathway, sequence and structural comparisons suggest that these sets of enzymes are not related to one another by divergent evolution. The common dinucleotide-binding domain found in the dehydrogenases (Figure 16.12) and the αβ barrels are the only major recurring elements.
We can speculate on the relationship between glycolysis and gluconeogenesis if we think of glycolysis as consisting of two segments: the metabolism of hexoses (the upper segment) and the metabolism of trioses (the lower segment). The enzymes of the upper segment are different in some species and are missing entirely in some archaea, whereas enzymes of the lower segment are quite conserved. In fact, four enzymes of the lower segment are present in all species. This lower part of the pathway is common to glycolysis and gluconeogenesis. This common part of the two pathways may be the oldest part, constituting the core to which the other steps were added. The upper part would have varied according to the sugars that were available to evolving organisms in particular niches. Interestingly, this core part of carbohydrate metabolism can generate triose precursors for ribose sugars, a component of RNA and a critical requirement for the RNA world. Thus, we are left with the unanswered question, Was the original core pathway used for energy conversion or biosynthesis?






 Isozymic forms of lactate dehydrogenase in different tissues catalyze the interconversions of pyruvate and lactate (Section 10.2). Lactate dehydrogenase is a tetramer of two kinds of 35-
Isozymic forms of lactate dehydrogenase in different tissues catalyze the interconversions of pyruvate and lactate (Section 10.2). Lactate dehydrogenase is a tetramer of two kinds of 35- The metabolism of glucose has ancient origins. Organisms living in the early biosphere depended on the anaerobic generation of energy until significant amounts of oxygen began to accumulate 2 billion years ago. Glycolytic enzymes were most likely derived independently rather than by gene duplication, because glycolytic enzymes with similar properties do not have similar amino acid sequences. Although there are four kinases and two isomerases in the pathway, sequence and structural comparisons suggest that these sets of enzymes are not related to one another by divergent evolution. The common dinucleotide-
The metabolism of glucose has ancient origins. Organisms living in the early biosphere depended on the anaerobic generation of energy until significant amounts of oxygen began to accumulate 2 billion years ago. Glycolytic enzymes were most likely derived independently rather than by gene duplication, because glycolytic enzymes with similar properties do not have similar amino acid sequences. Although there are four kinases and two isomerases in the pathway, sequence and structural comparisons suggest that these sets of enzymes are not related to one another by divergent evolution. The common dinucleotide-