10.2Isozymes Provide a Means of Regulation Specific to Distinct Tissues and Developmental Stages
Isozymes Provide a Means of Regulation Specific to Distinct Tissues and Developmental Stages
Isozymes, or isoenzymes, are enzymes that differ in amino acid sequence yet catalyze the same reaction. Typically, these enzymes display different kinetic parameters, such as KM, or respond to different regulatory molecules. They are encoded by different genes, which usually arise through gene duplication and divergence. Isozymes can often be distinguished from one another by physical properties such as electrophoretic mobility. Isoform is a more generic term used when the protein in question is not an enzyme.
The existence of isozymes permits the fine-
293
The M4 isozyme functions optimally in the anaerobic environment of hard-
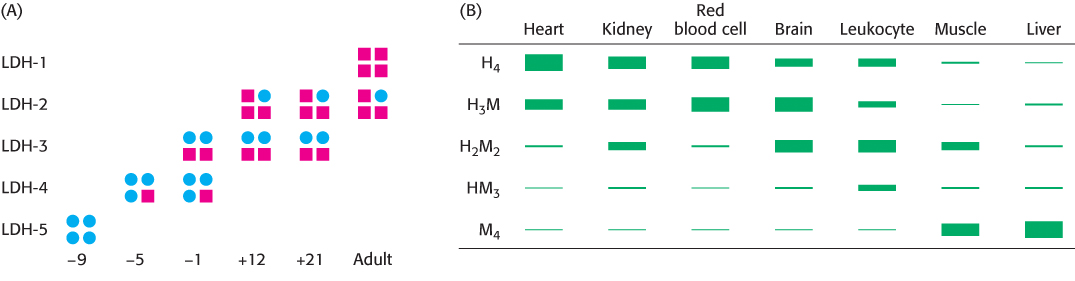
 The appearance of some isozymes in the blood is a sign of tissue damage, useful for clinical diagnosis. For instance, an increase in serum levels of H4 relative to H3M is an indication that a myocardial infarction, or heart attack, has damaged heart-
The appearance of some isozymes in the blood is a sign of tissue damage, useful for clinical diagnosis. For instance, an increase in serum levels of H4 relative to H3M is an indication that a myocardial infarction, or heart attack, has damaged heart-