8.3Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
The free-
A chemical reaction of substrate S to form product P goes through a transition state X‡ that has a higher free energy than does either S or P.
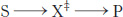
The double dagger denotes the transition state. The transition state is a transitory molecular structure that is no longer the substrate but is not yet the product. The transition state is the least-
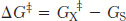
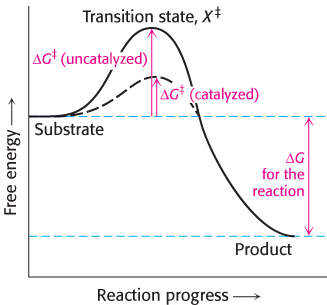
Note that the energy of activation, or ΔG‡, does not enter into the final ΔG calculation for the reaction, because the energy required to generate the transition state is released when the transition state forms the product. The activation-
222
“I think that enzymes are molecules that are complementary in structure to the activated complexes of the reactions that they catalyze, that is, to the molecular configuration that is intermediate between the reacting substances and the products of reaction for these catalyzed processes. The attraction of the enzyme molecule for the activated complex would thus lead to a decrease in its energy and hence to a decrease in the energy of activation of the reaction and to an increase in the rate of reaction.”
—Linus Pauling
Nature 161:707, 1948
One approach to understanding the increase in reaction rates achieved by enzymes is to assume that the transition state (X‡) and the substrate (S) are in equilibrium.
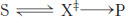
in which K‡ is the equilibrium constant for the formation of X‡ and v is the rate of formation of product from X‡. The rate of the reaction v is proportional to the concentration of X‡,
v ∝ [X‡],
because only X‡ can be converted into product. The concentration of X‡ at equilibrium is in turn related to the free-
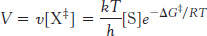
In this equation, k is Boltzmann’s constant, and h is Planck’s constant. The value of kT/h at 25°C is 6.6 × 1012 s−1. Suppose that the free energy of activation is 28.53 kJ mol−1 (6.82 kcal mol−1). If we were to substitute this value of ΔG in equation 7 (as shown in Table 8.3), this free-
Thus, we see the key to how enzymes operate: enzymes accelerate reactions by decreasing ΔG‡, the activation energy. The combination of substrate and enzyme creates a reaction pathway whose transition-
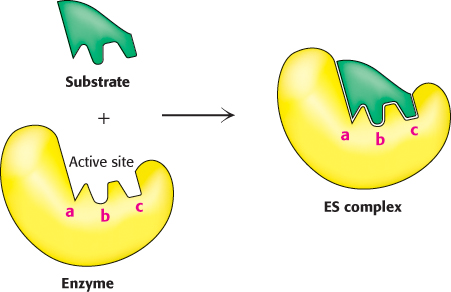
The formation of an enzyme–substrate complex is the first step in enzymatic catalysis
Much of the catalytic power of enzymes comes from their binding to and then altering the structure of the substrate to promote the formation of the transition state. Thus, the first step in catalysis is the formation of an enzyme–
What is the evidence for the existence of an enzyme–
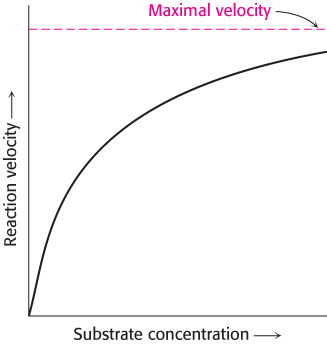
The first clue was the observation that, at a constant concentration of enzyme, the reaction rate increases with increasing substrate concentration until a maximal velocity is reached (Figure 8.4). In contrast, uncatalyzed reactions do not show this saturation effect. The fact that an enzyme-
catalyzed reaction has a maximal velocity suggests the formation of a discrete ES complex . At a sufficiently high substrate concentration, all the catalytic sites are filled, or saturated, and so the reaction rate cannot increase. Although indirect, the ability to saturate an enzyme with substrate is the most general evidence for the existence of ES complexes.223
The spectroscopic characteristics of many enzymes and substrates change on the formation of an ES complex. These changes are particularly striking if the enzyme contains a colored prosthetic group (Problem 39).
X-
ray crystallography has provided high-resolution images of substrates and substrate analogs bound to the active sites of many enzymes (Figure 8.5). In Chapter 9, we will take a close look at several of these complexes. 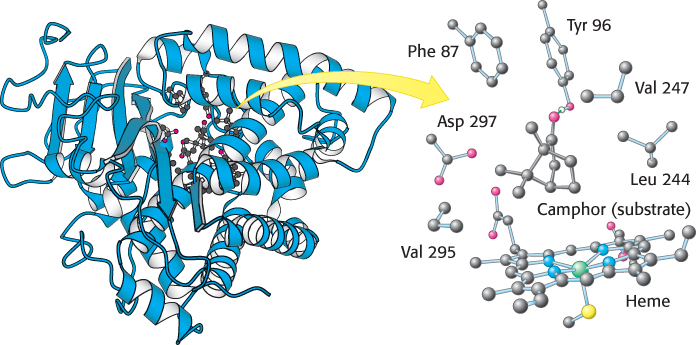
 FIGURE 8.5 Structure of an enzyme–
FIGURE 8.5 Structure of an enzyme–substrate complex. (Left) The enzyme cytochrome P450 is illustrated bound to its substrate camphor. (Right) Notice that, in the active site, the substrate is surrounded by residues from the enzyme. Note also the presence of a heme cofactor.[Drawn from 2CPP.pdb.]
The active sites of enzymes have some common features
The active site of an enzyme is the region that binds the substrates (and the cofactor, if any). It also contains the amino acid residues that directly participate in the making and breaking of bonds. These residues are called the catalytic groups. In essence, the interaction of the enzyme and substrate at the active site promotes the formation of the transition state. The active site is the region of the enzyme that most directly lowers the ΔG‡ of the reaction, thus providing the rate-
The active site is a three-
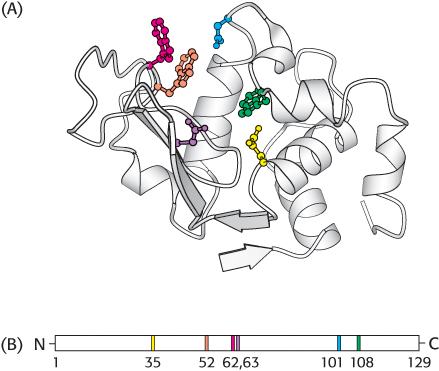
dimensional cleft, or crevice , formed by groups that come from different parts of the amino acid sequence: indeed, residues far apart in the amino acid sequence may interact more strongly than adjacent residues in the sequence, which may be sterically constrained from interacting with one another. In lysozyme, an enzyme that degrades the cell walls of some bacteria, the important groups in the active site are contributed by residues numbered 35, 52, 62, 63, 101, and 108 in the sequence of 129 amino acids (Figure 8.6).The active site takes up a small part of the total volume of an enzyme. Although most of the amino acid residues in an enzyme are not in contact with the substrate, the cooperative motions of the entire enzyme help to correctly position the catalytic residues at the active site. Experimental attempts to reduce the size of a catalytically active enzyme show that the minimum size requires about 100 amino acid residues. In fact, nearly all enzymes are made up of more than 100 amino acid residues, which gives them a mass greater than 10 kDa and a diameter of more than 25 Å, suggesting that all amino acids in the protein, not just those at the active site, are ultimately required to form a functional enzyme.
Active sites are unique microenvironments. In all enzymes of known structure, active sites are shaped like a cleft, or crevice, to which the substrates bind. Water is usually excluded unless it is a reactant. The nonpolar microenvironment of the cleft enhances the binding of substrates as well as catalysis. Nevertheless, the cleft may also contain polar residues, some of which may acquire special properties essential for substrate binding or catalysis. The internal positions of these polar residues are biologically crucial exceptions to the general rule that polar residues are located on the surface of proteins, exposed to water.
224
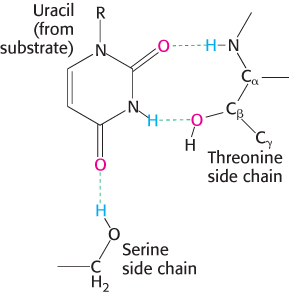
Substrates are bound to enzymes by multiple weak attractions. The noncovalent interactions in ES complexes are much weaker than covalent bonds, which have energies between −210 and −460 kJ mol−1 (between −50 and −110 kcal mol−1). In contrast, ES complexes usually have equilibrium constants that range from 10−2 to 10−8 M, corresponding to free energies of interaction ranging from about −13 to −50 kJ mol−1 (from −3 to −12 kcal mol−1). As discussed in Section 1.3, these weak reversible contacts are mediated by electrostatic interactions, hydrogen bonds, and van der Waals forces. Van der Waals forces become significant in binding only when numerous substrate atoms simultaneously come close to many enzyme atoms through the hydrophobic effect. Hence, the enzyme and substrate should have complementary shapes. The directional character of hydrogen bonds between enzyme and substrate often enforces a high degree of specificity, as seen in the RNA-
degrading enzyme ribonuclease (Figure 8.7). The specificity of binding depends on the precisely defined arrangement of atoms in an active site. Because the enzyme and the substrate interact by means of short-
range forces that require close contact, a substrate must have a matching shape to fit into the site. Emil Fischer proposed the lock- and- key analogy in 1890 (Figure 8.8), which was the model for enzyme– substrate interaction for several decades. We now know that enzymes are flexible and that the shapes of the active sites can be markedly modified by the binding of substrate, a process of dynamic recognition called induced fit (Figure 8.9). Moreover, the substrate may bind to only certain conformations of the enzyme, in what is called conformation selection. Thus, the mechanism of catalysis is dynamic, involving structural changes with multiple intermediates of both reactants and the enzyme.
225
The binding energy between enzyme and substrate is important for catalysis
Enzymes lower the activation energy, but where does the energy to lower the activation energy come from? Free energy is released by the formation of a large number of weak interactions between a complementary enzyme and its substrate. The free energy released on binding is called the binding energy. Only the correct substrate can participate in most or all of the interactions with the enzyme and thus maximize binding energy, accounting for the exquisite substrate specificity exhibited by many enzymes. Furthermore, the full complement of such interactions is formed only when the substrate is converted into the transition state. Thus, the maximal binding energy is released when the enzyme facilitates the formation of the transition state. The energy released by the interaction between the enzyme and the substrate can be thought of as lowering the activation energy. The interaction of the enzyme with the substrate and reaction intermediates is fleeting, with molecular movements resulting in the optimal alignment of functional groups at the active site so that maximum binding energy occurs only between the enzyme and the transition state, the least-