PROBLEMS
PROBLEMS
Question 22.1
Match’em. Match each term with its description.
|
(a) Triacylglycerol _____ (b) Perilipin _____ (c) Adipose triglyceride lipase _____ (d) Glucagon _____ (e) Acyl CoA synthetase _____ (f) Carnitine _____ (g) β-Oxidation pathway _____ (h) Enoyl CoA isomerase _____ (i) 2,4- (j) Methylmalonyl CoA mutase _____ (k) Ketone body _____ |
1. The enzyme that initiates lipid degradation 2. Activates fatty acids for degradation 3. Converts a cis-Δ3 double bond into a trans-Δ2 double bond 4. Reduces 2,4- 5. Storage form of fats 6. Required for entry into mitochondria 7. Requires vitamin B12 8. Acetoacetate 9. Means by which fatty acids are degraded 10. Stimulates lipolysis 11. Lipid- |
Question 22.2
After lipolysis. Write a balanced equation for the conversion of glycerol into pyruvate. Which enzymes are required in addition to those of the glycolytic pathway?
Question 22.3
Forms of energy. The partial reactions leading to the synthesis of acyl CoA (equations 1 and 2, in Section 22.2) are freely reversible. The equilibrium constant for the sum of these reactions is close to 1, meaning that the energy levels of the reactants and products are about equal, even though a molecule of ATP has been hydrolyzed. Explain why these reactions are readily reversible.
Question 22.4
Activation fee. The reaction for the activation of fatty acids before degradation is
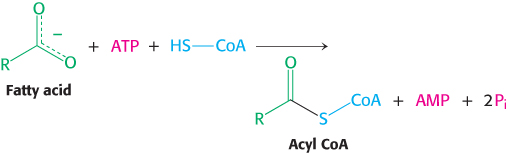
This reaction is quite favorable because the equivalent of two molecules of ATP is hydrolyzed. Explain why, from a biochemical bookkeeping point of view, the equivalent of two molecules of ATP is used despite the fact that the left side of the equation has only one molecule of ATP.
Question 22.5
Proper sequence. Place the following list of reactions or relevant locations in the β oxidation of fatty acids in the proper order.
Reaction with carnitine
Fatty acid in the cytoplasm
Activation of fatty acid by joining to CoA
Hydration
NAD+-linked oxidation
Thiolysis
Acyl CoA in mitochondrion
FAD-
linked oxidation.
Question 22.6
Remembrance of reactions past. We have encountered reactions similar to the oxidation, hydration, and oxidation reactions of fatty acid degradation earlier in our study of biochemistry. What other pathway employs this set of reactions?
Question 22.7
A phantom acetyl CoA? In the equation for fatty acid degradation shown here, only seven molecules of CoA are required to yield eight molecules of acetyl CoA. How is this difference possible?
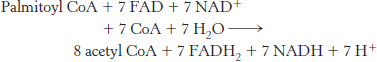
Question 22.8
Comparing yields. Compare the ATP yields from palmitic acid and palmitoleic acid.
Question 22.9
Counting ATPs 1. What is the ATP yield for the complete oxidation of C17 (heptadecanoic) fatty acid? Assume that the propionyl CoA ultimately yields oxaloacetate in the citric acid cycle.
675
Question 22.10
Sweet temptation. Stearic acid is a C18 fatty acid component of chocolate. Suppose you had a depressing day and decided to settle matters by gorging on chocolate. How much ATP would you derive from the complete oxidation of stearic acid to CO2?
Question 22.11
The best storage form. Compare the ATP yield from the complete oxidation of glucose, a six-
Question 22.12
From fatty acid to ketone body. Write a balanced equation for the conversion of stearate into acetoacetate.
Question 22.13
Generous, but not to a fault. Liver is the primary site of ketone-
Question 22.14
Counting ATPs 2. How much energy is attained with the complete oxidation of the ketone body d-3-
Question 22.15
Another view. Why might someone argue that the answer to problem 14 is wrong?
Question 22.16
An accurate adage. An old biochemistry adage is that fats burn in the flame of carbohydrates. What is the molecular basis of this adage?
Question 22.17
Refsum disease. Phytanic acid is a branched-
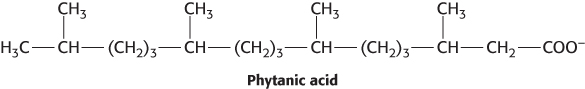
Why does phytanic acid accumulate?
What enzyme activity would you invent to prevent its accumulation?
Question 22.18
A hot diet. Tritium is a radioactive isotope of hydrogen and can be readily detected. A fully tritiated, six-
Question 22.19
Finding triacylglycerols in all the wrong places. Insulin-
Question 22.20
Match ’em. Match the terms with the descriptions.
|
(a) ATP- (b) Malic enzyme _____ (c) Malonyl CoA _____ (d) Acetyl CoA carboxylase _____ (e) Acyl carrier protein _____ (f) β-Ketoacyl synthase _____ (g) Palmitate _____ (h) Eicosanoids _____ (i) Arachidonate _____ (j) AMP- |
1. Helps to generate NADPH from NADH 2. Inactivates acetyl CoA carboxylase 3. Molecule on which fatty acids are synthesized 4. A precursor of prostaglandins 5. Activated acetyl CoA 6. The end product of fatty acid synthase 7. Fatty acids containing 20 carbon atoms 8. Catalyzes the committed step in fatty acid synthesis 9. Catalyzes the reaction of acetyl CoA and malonyl CoA 10. Generates cytoplasmic acetyl CoA |
Question 22.21
Counterpoint. Compare and contrast fatty acid oxidation and synthesis with respect to
site of the process
acyl carrier
reductants and oxidants
stereochemistry of the intermediates
direction of synthesis or degradation
organization of the enzyme system
Question 22.22
The fizz in fatty acid synthesis. During the initial in vitro studies on fatty acid synthesis, experiments were performed to determine which buffer would result in optimal activity. Bicarbonate buffer proved far superior to phosphate buffer. The researchers could not initially account for this result. From your perspective of many decades later, explain why the result is not surprising.
Question 22.23
A supple synthesis. Myristate, a saturated C14 fatty acid, is used as an emollient for cosmetics and topical medicinal preparations. Write a balanced equation for the synthesis of myristate.
Question 22.24
The cost of cleanliness. Lauric acid is a 12-
676
Question 22.25
Proper organization. Arrange the following steps in fatty acid synthesis in their proper order.
Dehydration
Condensation
Release of a C16 fatty acid
Reduction of a carbonyl group
Formation of malonyl ACP
Question 22.26
No access to assets. What would be the effect on fatty acid synthesis of a mutation in ATP-
Question 22.27
The truth and nothing but. True or False. If false, explain.
Biotin is required for fatty acid synthase activity.
The condensation reaction in fatty acid synthesis is powered by the decarboxylation of malonyl CoA.
Fatty acid synthesis does not depend on ATP.
Palmitate is the end product of fatty acid synthase.
All of the enzyme activities required for fatty acid synthesis in mammals are contained in a single polypeptide chain.
Fatty acid synthase in mammals is active as a monomer.
The fatty acid arachidonate is a precursor for signal molecules.
Acetyl CoA carboxylase is inhibited by citrate.
Question 22.28
Odd fat out. Suggest how fatty acids with odd numbers of carbons are synthesized.
Question 22.29
Labels. Suppose that you had an in vitro fatty acid-
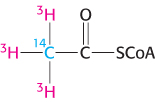
The ratio of 3H/14C is 3. What would the 3H/14C ratio be after the synthesis of palmitic acid (C16) with the use of the radioactive acetyl CoA?
Question 22.30
A tight embrace. Avidin, a glycoprotein found in eggs, has a high affinity for biotin. Avidin can bind biotin and prevent its use by the body. How might a diet rich in raw eggs affect fatty acid synthesis? What will be the effect on fatty acid synthesis of a diet rich in cooked eggs? Explain.
Question 22.31
Alpha or omega? Only one acetyl CoA molecule is used directly in fatty acid synthesis. Identify the carbon atoms in palmitic acid that were donated by acetyl CoA.
Question 22.32
Now you see it, now you don’t. Although 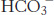 is required for fatty acid synthesis, its carbon atom does not appear in the product. Explain how this omission is possible.
is required for fatty acid synthesis, its carbon atom does not appear in the product. Explain how this omission is possible.
Question 22.33
It is all about communication. Why is citrate an appropriate inhibitor of phosphofructokinase?
Question 22.34
Tracing carbon atoms. Consider a cell extract that actively synthesizes palmitate. Suppose that a fatty acid synthase in this preparation forms one molecule of palmitate in about 5 minutes. A large amount of malonyl CoA labeled with 14C in each carbon atom of its malonyl unit is suddenly added to this system, and fatty acid synthesis is stopped a minute later by altering the pH. The fatty acids are analyzed for radioactivity. Which carbon atom of the palmitate formed by this system is more radioactive—
Question 22.35
An unaccepting mutant. The serine residues in acetyl CoA carboxylase that are the target of the AMP-
Question 22.36
Sources. For each of the following unsaturated fatty acids, indicate whether the biosynthetic precursor in animals is palmitoleate, oleate, linoleate, or linolenate.
18:1 cis-Δ11
18:3 cis-Δ6, Δ9, Δ12
20:2 cis-Δ11, Δ14
20:3 cis-Δ5, Δ8, Δ11
22:1 cis-Δ13
22:6 cis-Δ4, Δ7, Δ10, Δ13, Δ16, Δ19
Question 22.37
Driven by decarboxylation. What is the role of decarboxylation in fatty acid synthesis? Name another key reaction in a metabolic pathway that employs this mechanistic motif.
Question 22.38
Kinase surfeit. Suppose that a promoter mutation leads to the overproduction of protein kinase A in adipose cells. How might fatty acid metabolism be altered by this mutation?
Question 22.39
Blocked assets. The presence of a fuel molecule in the cytoplasm does not ensure that the fuel molecule can be effectively used. Give two examples of how impaired transport of metabolites between compartments leads to disease.
Question 22.40
Elegant inversion. Peroxisomes have an alternative pathway for oxidizing polyunsaturated fatty acids. They contain a hydratase that converts d-3-
Question 22.41
Arm in arm. Many eukaryotic enzymes, such as fatty acid synthase, are multifunctional proteins with multiple active sites covalently linked. What is the advantage of this arrangement?
Question 22.42
Covalent catastrophe. What is a potential disadvantage of having many catalytic sites together on one very long polypeptide chain?
Question 22.43
Missing acyl CoA dehydrogenases. A number of genetic deficiencies in acyl CoA dehydrogenases have been described. This deficiency presents early in life after a period of fasting. Symptoms include vomiting, lethargy, and sometimes coma. Not only are blood levels of glucose low (hypoglycemia), but starvation-
677
Question 22.44
Effects of clofibrate. High blood levels of triacylglycerides are associated with heart attacks and strokes. Clofibrate, a drug that increases the activity of peroxisomes, is sometimes used to treat patients with such a condition. What is the biochemical basis for this treatment?
Question 22.45
A different kind of enzyme. Figure 22.36 shows the response of acetyl CoA carboxylase to varying amounts of citrate. Explain this effect in light of the allosteric effects that citrate has on the enzyme. Predict the effects of increasing concentrations of palmitoyl CoA.
Mechanism Problems
Question 22.46
Variation on a theme. Thiolase is homologous in structure to the condensing enzyme. On the basis of this observation, propose a mechanism for the cleavage of 3-
Question 22.47
Two plus three to make four. Propose a reaction mechanism for the condensation of an acetyl unit with a malonyl unit to form an acetoacetyl unit in fatty acid synthesis.
Chapter Integration Problems
Question 22.48
Ill-
How would lack of carbohydrates affect your ability to utilize fats?
What would your breath smell like?
One of your best friends, after trying unsuccessfully to convince you to abandon this diet, makes you promise to consume a healthy dose of odd-
chain fatty acids. Does your friend have your best interests at heart? Explain.
Question 22.49
Fats to glycogen. An animal is fed stearic acid that is radioactively labeled with [14C]carbon. A liver biopsy reveals the presence of 14C-
Question 22.50
Enzyme deficit. Pyruvate dehydrogenase deficiency, a severe condition with a poor prognosis, results in neurological dysfunction and unremitting lactic acidosis. One treatment is placing the patients on a low carbohydrate/high fat diet.
Explain why the deficiency would be characterized by neurological dysfunction.
Why does lactic acidosis result?
Explain the rationale for the low carbohydrate/high fat diet.
Question 22.51
Right enzymes. Wrong location. Zwellweger syndrome is caused by a defect in enzyme importation into the peroxisomes. What disease of carbohydrate metabolism is caused by inappropriate cellular distribution of enzymes?
Data Interpretation Problem
Question 22.52
Mutant enzyme. Carnitine palmitoyl transferase I (CPTI) catalyzes the conversion of long-
What is the effect of the mutation on enzyme activity when the concentration of carnitine is varied (Graph A)? What are the KM and Vmax values for the wild-
type and mutant enzymes? 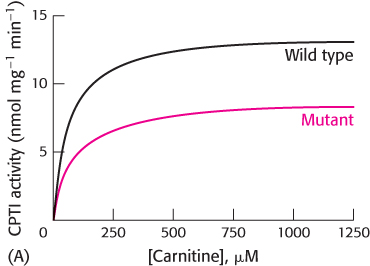
What is the effect when the experiment is repeated with varying concentrations of palmitoyl CoA (Graph B)? What are the KM andVmax values for the wild-
type and mutant enzymes? 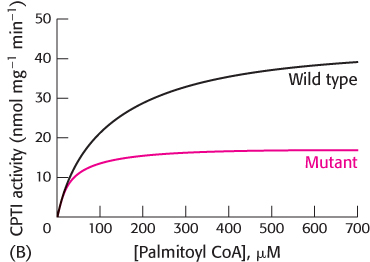
Graph C shows the inhibitory effect of malonyl CoA on the wild-
type and mutant enzymes. Which enzyme is more sensitive to malonyl CoA inhibition? 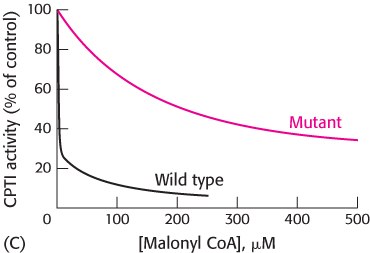
678
Suppose that the concentration of palmitoyl CoA = 100 μM, that of carnitine = 100 μM, and that of malonyl CoA = 10 μM. Under these conditions, what is the most prominent effect of the mutation on the properties of the enzyme?
What can you conclude about the role of glutamate 3 in CPTI function?
[Data from J. Shi, H. Zhu, D. N. Arvidson, and G. J. Woldegiorgis. J. Biol. Chem. 274:9421–
9426, 1999.]
Question 22.53
Partners. Colipase binds to lipid particles in the intestine and facilitates the subsequent binding and activation of pancreatic lipase. The lipase then hydrolyzes the lipids so that they can be absorbed by intestinal cells. Experiments were undertaken using site-
Figure A shows the results of two different amino acid substitutions on the ability of colipase to facilitate the binding of lipase to the lipid particles. In both panels, the blue circles show the control (no amino acid changes) data and the red circles the data with the altered amino acid. The first letter represents the normal amino acid, the number its position in the amino acid sequence, and the second letter the replacement amino acid.
What is the amino acid alteration in the upper panel?
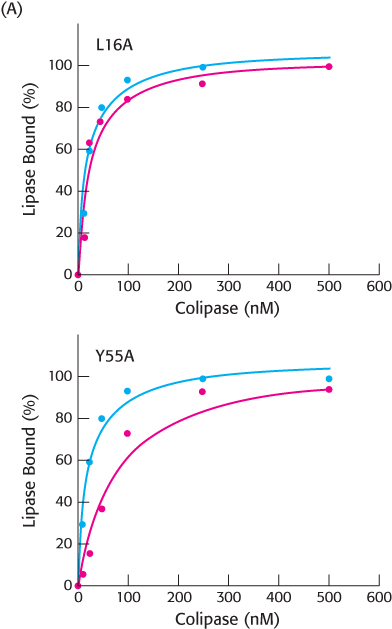
How does this mutation change the ability of colipase to bind the lipase to the lipid particle?
What is the amino acid substitution in the lower panel?
What is the effect of this substitution on the ability of the colipase to bind the lipase to the lipid particle?
Next, the activity of the bound lipase was examined, as shown in Figure B.
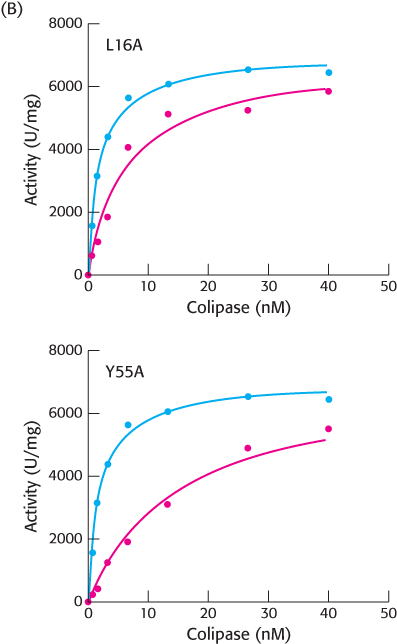 [Data from: Ross, L. E., Xiao, X., and Lowe, M. E. 2013. Identifi cation of amino acids in human colipase that mediate adsorption to lipid emulsions and mixed micelles. Biochim. Biophys. Acta 1831: 1052–
[Data from: Ross, L. E., Xiao, X., and Lowe, M. E. 2013. Identifi cation of amino acids in human colipase that mediate adsorption to lipid emulsions and mixed micelles. Biochim. Biophys. Acta 1831: 1052–1059.] What was the effect of the L16A mutation on lipase activity?
What does this suggest about the lipase binding and activation properties of the colipase?
What was the effect of the Y55A mutation on lipase activity?
Question 22.54
Nip it in the bud. Soraphen A is a natural antifungal agent isolated from a myxobacterium. Soraphen A is also a potent inhibitor of acetyl CoA carboxylase, the regulatory enzyme that initiates fatty acid synthesis. As discussed earlier, cancer cells must produce large amount of fatty acids to generate phospholipids for membrane synthesis. Thus, acetyl CoA carboxylase might be a target for anti-
679
Figure A shows the effect of various amounts of soraphen A on fatty acid synthesis as measured by the incorporation of radioactive acetate (14C) into fatty acids.
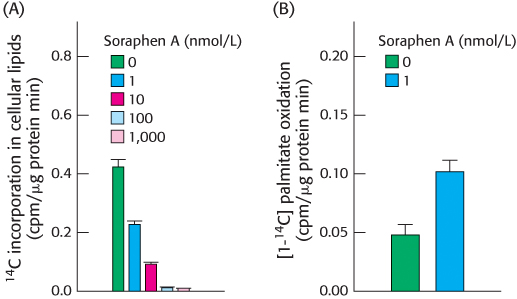
How did soraphen A alter fatty acid synthesis? How was synthesis affected by increasing concentrations of soraphen A?
Figure B shows the results obtained when fatty acid oxidation was measured, as the release of radioactive CO2 from added radioactive (14 C) palmitate, in the presence of soraphen A.
How did soraphen A alter fatty acid oxidation?
Explain the results obtained in B in light of the fact that soraphen A inhibits acetyl CoA carboxylase.
More experiments were undertaken to assess whether carboxylase inhibition did indeed prevent phospholipid synthesis. Again, cells were grown in the presence of radioactive acetate and phospholipids were subsequently isolated and the amount of 14C incorporated into the phospholipid was determined. The effect of soraphen A on phospholipid synthesis is shown in Figure C.
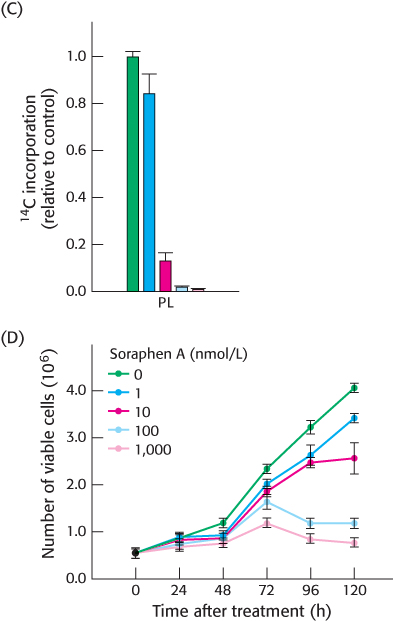 [Data from: Beckers, A., Organe, S., Timmermans, L., Scheys, K., Peeters, A., Brusselmans, K., Verhoeven, G., and Swinnen, J. V. 2007. Chemical inhibition of acetyl-
[Data from: Beckers, A., Organe, S., Timmermans, L., Scheys, K., Peeters, A., Brusselmans, K., Verhoeven, G., and Swinnen, J. V. 2007. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 67: 8180– 8187.] Did the inhibition of carboxylase by the drug alter phospholipid synthesis?
How might phospholipid synthesis affect cell viability?
Finally, as shown in Figure D, experiments were performed to determine if the drug inhibits cancer growth in vitro by determining the number of cancer cells surviving upon exposure to soraphen A.
How did soraphen A affect cancer cell viability?
680